Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury
- PMID: 20030560
- PMCID: PMC2867631
- DOI: 10.1089/neu.2009.0966
Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury
Abstract
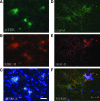
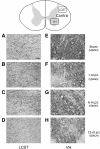
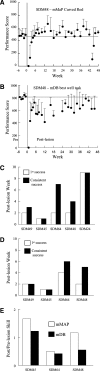
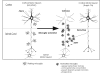
Recovery of fine motor skills after traumatic brain injury (TBI) is variable, with some patients showing progressive improvements over time while others show poor recovery. We therefore studied possible cellular mechanisms accompanying the recovery process in a non-human primate model system, in which the lateral frontal motor cortex areas controlling the preferred upper limb were unilaterally lesioned, and the animals eventually regained fine hand motor function. Immunohistochemical staining of the cervical spinal cord, the site of compensatory sprouting and degeneration of corticospinal axons, showed profound increases in immunoreactivities for major histocompatibility complex class II molecule (MHC-II) and extracellular signal-regulated kinases (ERK1/2) up to 12 months post lesion, particularly within the lateral corticospinal tract (LCST). Double immunostaining demonstrated that phosphorylated ERK1/2 colocalized within the MCH-II + microglia, suggesting a trophic role of long-term microglia activation after TBI at the site of compensatory sprouting. Active sprouting was observed in the LCST as well as in the spinal gray matter of the lesioned animals, as illustrated by increases in growth associated protein 43. Upregulation of Nogo receptor and glutamate transporter expression was also observed in this region after TBI, suggesting possible mechanisms for controlling aberrant sprouting and/or synaptic formation en route and interstitial glutamate concentration changes at the site of axon degeneration, respectively. Taken together, these changes in the non-human primate spinal cord support a long-term trophic/tropic role for reactive microglia, in particular, during functional and structural recovery after TBI.
Figures









Similar articles
-
Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat.Neurorehabil Neural Repair. 2008 May-Jun;22(3):262-78. doi: 10.1177/1545968307308550. Epub 2007 Nov 30. Neurorehabil Neural Repair. 2008. PMID: 18056009 Free PMC article.
-
Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury.J Neurotrauma. 2007 Nov;24(11):1719-42. doi: 10.1089/neu.2007.0377. J Neurotrauma. 2007. PMID: 18001202
-
Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury.Brain. 2020 Jun 1;143(6):1697-1713. doi: 10.1093/brain/awaa116. Brain. 2020. PMID: 32375169 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system.Ann Med. 2005;37(8):556-67. doi: 10.1080/07853890500407520. Ann Med. 2005. PMID: 16338758 Review.
Cited by
-
Microglia during development and aging.Pharmacol Ther. 2013 Sep;139(3):313-26. doi: 10.1016/j.pharmthera.2013.04.013. Epub 2013 Apr 30. Pharmacol Ther. 2013. PMID: 23644076 Free PMC article. Review.
-
Extensive somatosensory and motor corticospinal sprouting occurs following a central dorsal column lesion in monkeys.J Comp Neurol. 2018 Oct 15;526(15):2373-2387. doi: 10.1002/cne.24491. Epub 2018 Sep 25. J Comp Neurol. 2018. PMID: 30014461 Free PMC article.
-
Minimal forced use without constraint stimulates spontaneous use of the impaired upper extremity following motor cortex injury.Exp Brain Res. 2010 May;202(3):529-42. doi: 10.1007/s00221-010-2157-y. Epub 2010 Jan 28. Exp Brain Res. 2010. PMID: 20107980 Free PMC article.
-
Visualizing Wallerian degeneration in the corticospinal tract after sensorimotor cortex ischemia in mice.Neural Regen Res. 2024 Mar;19(3):636-641. doi: 10.4103/1673-5374.380903. Neural Regen Res. 2024. PMID: 37721295 Free PMC article.
-
Functional recovery following motor cortex lesions in non-human primates: experimental implications for human stroke patients.J Integr Neurosci. 2011 Sep;10(3):353-84. doi: 10.1142/S0219635211002737. J Integr Neurosci. 2011. PMID: 21960307 Free PMC article. Review.
References
-
- Agrawal S.K. Theriault E. Fehlings M. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J. Neurotrauma. 1998;15:929–941. - PubMed
-
- Ashwal S. Holshouser B. Tong K. Serna T. Osterdock R. Gross M. Kido D. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J. Neurotrauma. 2004;21:1539–1552. - PubMed
-
- Batchelor P.E. Liberatore G.T. Porritt M.J. Donnan G.A. Howells D.W. Inhibition of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression reduces dopaminergic sprouting in the injured striatum. Eur. J. Neurosci. 2000;12:3462–3468. - PubMed
-
- Batchelor P.E. Porritt M.J. Martinello P. Parish C.L. Liberatore G.T. Donnan G.A. Howells D.W. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol. Cell Neurosci. 2002;21:436–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

