The Brief Pain Inventory and its "pain at its worst in the last 24 hours" item: clinical trial endpoint considerations
- PMID: 20030743
- PMCID: PMC3806650
- DOI: 10.1111/j.1526-4637.2009.00774.x
The Brief Pain Inventory and its "pain at its worst in the last 24 hours" item: clinical trial endpoint considerations
Abstract
Context: In 2006, the United States Food and Drug Administration (FDA) released a draft Guidance for Industry on the use of patient-reported outcomes (PRO) Measures in Medical Product Development to Support Labeling Claims. This draft guidance outlines psychometric aspects that should be considered when designing a PRO measure, including conceptual framework, content validity, construct validity, reliability, and the ability to detect clinically meaningful score changes. When finalized, it may provide a blueprint for evaluations of PRO measures that can be considered by sponsors and investigators involved in PRO research and drug registration trials.
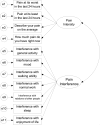
Objective: In this review we examine the short form of the Brief Pain Inventory (BPI) and particularly the "pain at its worst in the last 24 hours" item in the context of the FDA draft guidance, to assess its utility in clinical trials that include pain as a PRO endpoint.
Results and conclusions: After a systematic evaluation of the psychometric aspects of the BPI, we conclude that the BPI and its "pain at its worst in the last 24 hours" item generically satisfy most key recommendations outlined in the draft guidance for assessing a pain-reduction treatment effect. Nonetheless, when the BPI is being considered for assessment of pain endpoints in a registration trial, sponsors and investigators should consult with the appropriate FDA division early during research design to discuss whether there is sufficient precedent to use the instrument in the population of interest or whether additional evaluations of measurement properties are advisable.
Figures
References
-
- Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. 2008;139:485–493. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N Engl J Med. 2004;351(15):1502–1512. - PubMed
-
- Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy - Prostate: Results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12(1):124–129. - PubMed
-
- Cleeland CS. Research in cancer pain. What we know and what we need to know. Cancer. 1991;67(suppl 3):823–827. - PubMed
-
- Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical