A case of polymicrogyria in macaque monkey: impact on anatomy and function of the motor system
- PMID: 20030837
- PMCID: PMC2807873
- DOI: 10.1186/1471-2202-10-155
A case of polymicrogyria in macaque monkey: impact on anatomy and function of the motor system
Abstract
Background: Polymicrogyria is a malformation of the cerebral cortex often resulting in epilepsy or mental retardation. It remains unclear whether this pathology affects the structure and function of the corticospinal (CS) system. The anatomy and histology of the brain of one macaque monkey exhibiting a spontaneous polymicrogyria (PMG monkey) were examined and compared to the brain of normal monkeys. The CS tract was labelled by injecting a neuronal tracer (BDA) unilaterally in a region where low intensity electrical microstimulation elicited contralateral hand movements (presumably the primary motor cortex in the PMG monkey).
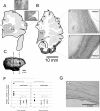
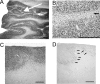
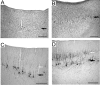
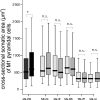
Results: The examination of the brain showed a large number of microgyri at macro- and microscopic levels, covering mainly the frontoparietal regions. The layered cortical organization was locally disrupted and the number of SMI-32 stained pyramidal neurons in the cortical layer III of the presumed motor cortex was reduced. We compared the distribution of labelled CS axons in the PMG monkey at spinal cervical level C5. The cumulated length of CS axon arbors in the spinal grey matter was not significantly different in the PMG monkey. In the red nucleus, numerous neurons presented large vesicles. We also assessed its motor performances by comparing its capacity to execute a complex reach and grasp behavioral task. The PMG monkey exhibited an increase of reaction time without any modification of other motor parameters, an observation in line with a normal CS tract organisation.
Conclusion: In spite of substantial cortical malformations in the frontal and parietal lobes, the PMG monkey exhibits surprisingly normal structure and function of the corticospinal system.
Figures



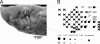
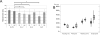
Similar articles
-
Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury in rhesus monkey.J Comp Neurol. 2015 Mar 1;523(4):669-97. doi: 10.1002/cne.23703. Epub 2014 Dec 19. J Comp Neurol. 2015. PMID: 25349147 Free PMC article.
-
A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex.J Neurotrauma. 2005 Jun;22(6):703-17. doi: 10.1089/neu.2005.22.703. J Neurotrauma. 2005. PMID: 15941378
-
Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the nonlesioned hemisphere in monkey (Macaca mulatta).J Comp Neurol. 2016 Feb 1;524(2):380-407. doi: 10.1002/cne.23861. Epub 2015 Aug 18. J Comp Neurol. 2016. PMID: 26224429 Free PMC article.
-
Effects of rehabilitative training on recovery of hand motor function: a review of animal studies.Neurosci Res. 2014 Jan;78:9-15. doi: 10.1016/j.neures.2013.09.008. Epub 2013 Sep 27. Neurosci Res. 2014. PMID: 24080147 Review.
-
Training-induced recovery of manual dexterity after a lesion in the motor cortex.Keio J Med. 2010;59(1):4-9. doi: 10.2302/kjm.59.4. Keio J Med. 2010. PMID: 20375652 Review.
Cited by
-
Ipsilateral corticotectal projections from the primary, premotor and supplementary motor cortical areas in adult macaque monkeys: a quantitative anterograde tracing study.Eur J Neurosci. 2017 Oct;46(8):2406-2415. doi: 10.1111/ejn.13709. Epub 2017 Oct 9. Eur J Neurosci. 2017. PMID: 28921678 Free PMC article.
References
-
- Guerrini R, Dravet C, Raybaud C, Roger J, Bureau M, Battaglia A, Livet MO, Colicchio G, Robain O. Neurological findings and seizure outcome in children with bilateral opercular macrogyric-like changes detected by MRI. Dev Med Child Neurol. 1992;34:694–705. - PubMed
-
- Chang BS, Piao X, Giannini C, Cascino GD, Scheffer I, Woods CG, Topcu M, Tezcan K, Bodell A, Leventer RJ, Barkovich AJ, Grant PE, Walsh CA. Bilateral generalized polymicrogyria (BGP): a distinct syndrome of cortical malformation. Neurology. 2004;62:1722–1728. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

