Treatment outcomes and plasma level of ritonavir-boosted lopinavir monotherapy among HIV-infected patients who had NRTI and NNRTI failure
- PMID: 20030841
- PMCID: PMC2805683
- DOI: 10.1186/1742-6405-6-30
Treatment outcomes and plasma level of ritonavir-boosted lopinavir monotherapy among HIV-infected patients who had NRTI and NNRTI failure
Abstract
Background: Different strategies of ritonavir-boosted lopinavir monotherapy have been explored; however, data regarding salvage therapy among HIV-infected patients who failed nucleoside reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) is still limited.
Methods: A prospective study was conducted among HIV-infected patients who failed NNRTI-based antiretroviral therapy with M184V, TAMs, and NNRTI mutations, and were naïve to protease inhibitor. LPV/r at 400/100 mg and lamivudine 150 mg were given twice daily. CD4 and HIV-1 RNA were monitored at week 0, 12, 24, and 48. LPV Cmin was assayed for the first 14 patients using HPLC.
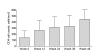
Results: There were 40 patients with a mean age of 37 years and 70% were male. Median (IQR) baseline CD4 was 123 (37-245) cells/mm(3) and median (IQR) HIV-1 RNA was 55,800 (9,670-100,000) copies/mL. By intend-to-treat analysis, 30 (75%) and 24 (60%) patients achieved HIV-1 RNA at <400 and <50 copies/mL, respectively. In as-treated analysis, the corresponding rates were 29 (83%) and 23 (67%), respectively. Low-level viral rebound was found in 6 (15%) patients at week 48. Medians CD4 at week 12, 24, 36 and 48 were 249, 283, 307, and 351 cells/mm(3) and significantly changed from baseline (all, P < 0.05). At 6 and 12 weeks, median (min-max) LPV Cmin was 6.52 (1.62-11.64) mg/L and 5.79 (0.75-16.31) mg/L, respectively. There were increments of mean total cholesterol and triglyceride at 48 weeks from baseline (P < 0.05).
Conclusion: LPV/r monotherapy with recycled lamivudine can maintain virological suppression in a substantial proportion of patients failing NNRTI-based regimen and provides adequate plasma concentrations of LPV although the incidence of low-level viremia is relatively high.
Figures
References
-
- Scaling up Antiretroviral Therapy in Resource-limited Settings: Treatment Guidelines for a public Health Approach. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); 2006 revision;
-
- Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Piyavong B, Chumpathat N, Chantratita W. Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis. 2007;44(3):447–452. doi: 10.1086/510745. - DOI - PubMed
-
- Hirsch MS, Gunthard HF, Schapiro JM, Brun-Vezinet F, Clotet B, Hammer SM, Johnson VA, Kuritzkes DR, Mellors JW, Pillay D. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47(2):266–285. doi: 10.1086/589297. - DOI - PubMed
-
- Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. U. S. Department of Health and Human Services (DHHS) http://AIDSinfo.nih.gov - PubMed
LinkOut - more resources
Full Text Sources
Research Materials