Rationale and design of WEBCARE: a randomized, controlled, web-based behavioral intervention trial in cardioverter-defibrillator patients to reduce anxiety and device concerns and enhance quality of life
- PMID: 20030843
- PMCID: PMC2813226
- DOI: 10.1186/1745-6215-10-120
Rationale and design of WEBCARE: a randomized, controlled, web-based behavioral intervention trial in cardioverter-defibrillator patients to reduce anxiety and device concerns and enhance quality of life
Abstract
Background: The implantable cardioverter defibrillator (ICD) is generally well accepted, but 25-33% of patients experience clinical levels of anxiety, depression, and impaired quality of life (QoL) following implantation. Few trials in ICD patients have investigated whether behavioral intervention may mitigate the development of these adjustment problems. We present the rationale and study design of the WEB-based distress management program for implantable CARdioverter dEfibrillator patients (WEBCARE) trial.
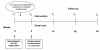
Methods: WEBCARE is a multi-center, multi-disciplinary, randomized, controlled behavioral intervention trial designed to examine the effectiveness of a web-based approach in terms of reducing levels of anxiety and device concerns and enhancing QoL. Consecutive patients hospitalized for the implantation of an ICD will be approached for study participation while in hospital and randomized to the intervention arm (n = 175) versus usual care (n = 175) at baseline (5-10 days post implantation). Patients will complete assessments of patient-centered outcomes at baseline, 14, 26, and 52 weeks after implantation. Patients randomized to the intervention arm will receive a 12-week web-based behavioral intervention starting 2 weeks after implantation. Primary endpoints include (i(i)) patient-centered outcomes (i.e., anxiety, depression, ICD acceptance, QoL); (i(ii)) health care utilization; and (i(iii)) cost-effectiveness. All primary endpoints will be assessed with standardized and validated disease-specific or generic questionnaires. Secondary endpoints include (ii(i)) cortisol awakening response; and (ii(ii)) ventricular arrhythmias.
Discussion: WEBCARE will show whether a behavioral intervention using a web-based approach is feasible and effective in reducing anxiety and ICD concerns and improving QoL in ICD patients.
Trial registration: http://www.ClinicalTrials.gov. Identifier: NCT00895700.
Figures
References
-
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. - DOI - PubMed
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. - DOI - PubMed