The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone
- PMID: 20030854
- PMCID: PMC2803175
- DOI: 10.1186/1745-6215-10-121
The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone
Abstract
Background: The standard of care for metastatic gastric cancer (MGC) is systemic chemotherapy which leads to a median survival of 6-15 months. Survival beyond 3 years is rare. For selected groups of patients with limited MGC, retrospective studies have shown improved overall survival following gastrectomy and metastasectomies including peritoneal stripping with continuous hyperthermic peritoneal perfusion (CHPP), liver resection, and pulmonary resection. Median survival after liver resection for MGC is up to 34 months, with a five year survival rate of 24.5%. Similarly, reported median survival after pulmonary resection of MGC is 21 months with long term survival of greater than 5 years a possibility. Several case reports and small studies have documented evidence of long-term survival in select individuals who undergo CHPP for MGC.
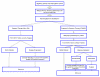
Design: The GYMSSA trial is a prospective randomized trial for patients with MGC. It is designed to compare two therapeutic approaches: gastrectomy with metastasectomy plus systemic chemotherapy (GYMS) versus systemic chemotherapy alone (SA). Systemic therapy will be composed of the FOLFOXIRI regimen. The aim of the study is to evaluate overall survival and potential selection criteria to determine those patients who may benefit from surgery plus systemic therapy. The study will be conducted by the Surgery Branch at the National Cancer Institute (NCI), National Institutes of Health (NIH) in Bethesda, Maryland. Surgeries and followup will be done at the NCI, and chemotherapy will be given by either the local oncologist or the medical oncology branch at NCI.
Trial registration: ClinicalTrials.gov ID. NCT00941655.
References
-
- Globocan 2002 database. http://www-dep.iarc.fr
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical