Downregulation of protein kinase C-alpha enhances intracellular survival of Mycobacteria: role of PknG
- PMID: 20030858
- PMCID: PMC2816201
- DOI: 10.1186/1471-2180-9-271
Downregulation of protein kinase C-alpha enhances intracellular survival of Mycobacteria: role of PknG
Abstract
Background: Intracellular trafficking of mycobacteria is comprehensively dependent on the unusual regulation of host proteins. Recently, we have reported that infection of macrophages by Mycobacterium tuberculosis H37Rv (Rv) selectively downregulates the expression of PKCalpha while infection by Mycobacterium smegmatis (MS) does not.
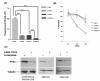
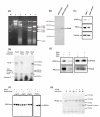
Results: Based on our earlier study, we have extrapolated for the first time that knockdown of PKCalpha, impairs phagocytosis of mycobacteria by macrophages while their intracellular survival is drastically increased. Mycobacterium bovis BCG (BCG) and Mycobacterium tuberculosis H37Ra (Ra) have also been shown to downregulate the expression of PKCalpha during the infection. Since PknG is uniquely expressed in BCG, Ra, Rv but not in MS and has been reported to promote intracellular survival of mycobacteria, led us to believe that PknG may be involved in such downregulation of PKCalpha. THP-1 cells infected with recombinant MS expressing PknG (MS-G), showed significant reduction in PKCalpha expression. In normal THP-1 cells survival of MS-G was enhanced as compared to MS, while their behavior in PKCalpha deficient cells could not be distinguished. The results strongly demonstrate that pathogenic mycobacteria recognize and then inhibit PKCalpha to circumvent phagocytosis and the hostile environment of macrophages. We emphasize that, this inhibition is controlled by PknG.
Conclusions: All together, our data reveal a mechanism that shows substantial interdependence of PKCalpha with PknG, in sustaining mycobacterial infection.
Figures

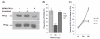
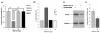

Similar articles
-
Differential expression of a virulence factor in pathogenic and non-pathogenic mycobacteria.Mol Microbiol. 2009 Apr;72(1):41-52. doi: 10.1111/j.1365-2958.2009.06612.x. Epub 2009 Feb 2. Mol Microbiol. 2009. PMID: 19210624 Free PMC article.
-
PknG supports mycobacterial adaptation in acidic environment.Mol Cell Biochem. 2018 Jun;443(1-2):69-80. doi: 10.1007/s11010-017-3211-x. Epub 2017 Nov 9. Mol Cell Biochem. 2018. PMID: 29124568
-
A Bumpy Ride of Mycobacterial Phagosome Maturation: Roleplay of Coronin1 Through Cofilin1 and cAMP.Front Immunol. 2021 Sep 23;12:687044. doi: 10.3389/fimmu.2021.687044. eCollection 2021. Front Immunol. 2021. PMID: 34630380 Free PMC article.
-
Combating Tuberculosis via Restoring the Host Immune Capacity by Targeting M. tb Kinases and Phosphatases.Int J Mol Sci. 2024 Nov 20;25(22):12481. doi: 10.3390/ijms252212481. Int J Mol Sci. 2024. PMID: 39596546 Free PMC article. Review.
-
To catch a killer. What can mycobacterial models teach us about Mycobacterium tuberculosis pathogenesis?Curr Opin Microbiol. 2010 Feb;13(1):86-92. doi: 10.1016/j.mib.2009.11.006. Epub 2009 Dec 23. Curr Opin Microbiol. 2010. PMID: 20036184 Free PMC article. Review.
Cited by
-
Surface Glycans Regulate Salmonella Infection-Dependent Directional Switch in Macrophage Galvanotaxis Independent of NanH.Infect Immun. 2022 Jan 25;90(1):e0051621. doi: 10.1128/IAI.00516-21. Epub 2021 Oct 18. Infect Immun. 2022. PMID: 34662214 Free PMC article.
-
Rv3080c regulates the rate of inhibition of mycobacteria by isoniazid through FabD.Mol Cell Biochem. 2013 Feb;374(1-2):149-55. doi: 10.1007/s11010-012-1514-5. Epub 2012 Nov 24. Mol Cell Biochem. 2013. PMID: 23180244
-
Sleeping with the enemy: how intracellular pathogens cope with a macrophage lifestyle.PLoS Pathog. 2012;8(3):e1002551. doi: 10.1371/journal.ppat.1002551. Epub 2012 Mar 22. PLoS Pathog. 2012. PMID: 22457616 Free PMC article. No abstract available.
-
Protein kinase G-a key regulator of pathogenesis in Mycobacterium tuberculosis infection.Arch Microbiol. 2025 May 28;207(7):154. doi: 10.1007/s00203-025-04355-7. Arch Microbiol. 2025. PMID: 40434519 Review.
-
Mycobacterium tuberculosis protein kinase G acts as an unusual ubiquitinating enzyme to impair host immunity.EMBO Rep. 2021 Jun 4;22(6):e52175. doi: 10.15252/embr.202052175. Epub 2021 May 2. EMBO Rep. 2021. PMID: 33938130 Free PMC article.
References
-
- Malik ZA, Iyer SS, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J Immunol. 2001;166:3392–3401. - PubMed
-
- Rao KMK. MAP kinase activation in macrophages. J Leukoc Biol. 2001;69:3–10. - PubMed
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor T, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

