In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels
- PMID: 20031638
- PMCID: PMC2801877
- DOI: 10.1161/CIRCGENETICS.108.847814
In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels
Abstract
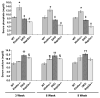
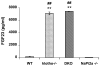
Background: Klotho-knockout mice (klotho(-/-)) have increased renal expression of sodium/phosphate cotransporters (NaPi2a), associated with severe hyperphosphatemia. Such serum biochemical changes in klotho(-/-) mice lead to extensive soft-tissue anomalies and vascular calcification. To determine the significance of increased renal expression of the NaPi2a protein and concomitant hyperphosphatemia and vascular calcification in klotho(-/-) mice, we generated klotho and NaPi2a double-knockout (klotho(-/-)/NaPi2a(-/-)) mice.
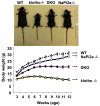
Methods and results: Genetic inactivation of NaPi2a activity from klotho(-/-) mice reversed the severe hyperphosphatemia to mild hypophosphatemia or normophosphatemia. Importantly, despite significantly higher serum calcium and 1,25-dihydroxyvitamin D levels in klotho(-/-)/NaPi2a(-/-) mice, the vascular and soft-tissue calcifications were reduced. Extensive soft-tissue anomalies and cardiovascular calcification were consistently noted in klotho(-/-) mice by 6 weeks of age; however, these vascular and soft-tissue abnormalities were absent even in 12-week-old double-knockout mice. Klotho(-/-)/NaPi2a(-/-) mice also regained body weight and did not develop the generalized tissue atrophy often noted in klotho(-/-) single-knockout mice.
Conclusions: Our in vivo genetic manipulation studies have provided compelling evidence for a pathological role of increased NaPi2a activities in regulating abnormal mineral ion metabolism and soft-tissue anomalies in klotho(-/-) mice. Notably, our results suggest that serum phosphate levels are the important in vivo determinant of calcification and that lowering serum phosphate levels can reduce or eliminate soft-tissue and vascular calcification, even in presence of extremely high serum calcium and 1,25-dihydroxyvitamin D levels. These in vivo observations have significant clinical importance and therapeutic implications for patients with chronic kidney disease with cardiovascular calcification.
Figures



References
-
- Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–101. - PubMed
-
- Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–5. - PubMed
-
- Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3. - PubMed
-
- Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

