Sexual conflict and the gender load: correlated evolution between population fitness and sexual dimorphism in seed beetles
- PMID: 20031994
- PMCID: PMC2871940
- DOI: 10.1098/rspb.2009.2026
Sexual conflict and the gender load: correlated evolution between population fitness and sexual dimorphism in seed beetles
Abstract
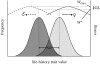
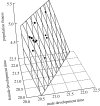
Although males and females share much of the same genome, selection is often distinct in the two sexes. Sexually antagonistic loci will in theory cause a gender load in populations, because sex-specific selection on a given trait in one sex will compromise the adaptive evolution of the same trait in the other sex. However, it is currently not clear whether such intralocus sexual conflict (ISC) represents a transient evolutionary state, where conflict is rapidly resolved by the evolution of sexual dimorphism (SD), or whether it is a more chronic impediment to adaptation. All else being equal, ISC should manifest itself as correlated evolution between population fitness and SD in traits expressed in both sexes. However, comparative tests of this prediction are problematic and have been unfeasible. Here, we assess the effects of ISC by comparing fitness and SD across distinct laboratory populations of seed beetles that should be well adapted to a shared environment. We show that SD in juvenile development time, a key life-history trait with a history of sexually antagonistic selection in this model system, is positively related to fitness. This effect is due to a correlated evolution between population fitness and development time that is positive in females but negative in males. Loosening the genetic bind between the sexes has evidently allowed the sexes to approach their distinct adaptive peaks.
Figures

References
-
- Arnqvist G., Rowe L.2002Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789 (doi:10.1038/415787a) - DOI - PubMed
-
- Arnqvist G., Rowe L.2005Sexual conflict Princeton, NJ: Princeton University Press
-
- Bedhomme S., Chippindale A. K.2008Irreconcilable differences: when sexual dimorphism fails to resolve sexual conflict. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Szekely T.), pp. 185–194 Oxford, UK: Oxford University Press
-
- Bieri J., Kawecki T. J.2003Genetic architecture of differences between populations of cowpea weevil (Callosobruchus maculatus) evolved in the same environment. Evolution 57, 274–287 (doi:10.1111/j.0014-3820.2003.tb00262.x) - DOI - PubMed
-
- Bilde T., Friberg U., Maklakov A. A., Fry J. D., Arnqvist G.2008The genetic architecture of fitness in a seed beetle: assessing the potential for indirect genetic benefits of female choice. BMC Evol. Biol. 8, 295 (doi:10.1186/1471-2148-8-295) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
