Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury
- PMID: 20032114
- PMCID: PMC2838607
- DOI: 10.1152/ajprenal.00683.2009
Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury
Abstract
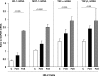
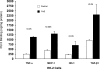
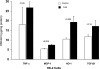
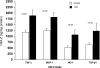
Rhabdomyolysis (Fe)-induced acute renal failure (ARF) causes renal inflammation, and, with repetitive insults, progressive renal failure can result. To gain insights into these phenomena, we assessed the impact of a single episode of glycerol-induced rhabdomyolysis on proinflammatory/profibrotic [TNF-alpha, monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor-beta1 (TGF-beta1)] gene expression and the time course of these changes. CD-1 mice were studied 1-7 days after glycerol injection. Normal mice served as controls. RNA polymerase II (Pol II) binding to the TNF-alpha, MCP-1, and TGF-beta1 genes, "gene-activating" histone modifications [histone 3 lysine 4 (H3K4) trimethylation (H3K4m3) and histone 2 variant H2A.Z], and cognate mRNA levels were assessed. Results were contrasted to changes in anti-inflammatory heme oxygenase-1 (HO-1). Glycerol produced severe ARF (blood urea nitrogen approximately 150-180 mg/dl) followed by marked improvement by day 7 (blood urea nitrogen approximately 40 mg/dl). Early increases in TNF-alpha, MCP-1, and TGF-beta1 mRNAs, Pol II gene binding, and H3K4m3/H2A.Z levels were observed. These progressed with time, despite resolution of azotemia. Comparable early HO-1 changes were observed. However, HO-1 mRNA normalized by day 7, and progressive Pol II binding/histone alterations did not occur. Fe-mediated injury to cultured proximal tubule (HK-2) cells recapitulated these in vivo results. Hence, this in vitro model was used for mechanistic assessments. On the basis of these studies, it was determined that 1) the H3K4m3/H2A.Z increases are early events (i.e., they precede mRNA increases), 2) subsequent mRNA elevations reflect transcription, not mRNA stabilization (actinomycin D assessments), and 3) increased transcription, per se, helps sustain elevated H2A.Z levels. We conclude that 1) Fe/glycerol-induced tubular injury causes sustained proinflammatory gene activation, 2) decreasing HO-1 expression, as reflected by mRNA levels, may facilitate this proinflammatory state, and 3) gene-activating histone modifications are early injury events and progressively increase at selected proinflammatory genes. Thus they may help sustain a proinflammatory state, despite resolving ARF.
Figures
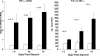

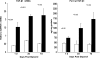

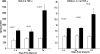
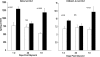
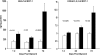
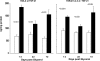



Similar articles
-
Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes.Am J Physiol Renal Physiol. 2009 May;296(5):F1032-41. doi: 10.1152/ajprenal.00061.2009. Epub 2009 Mar 4. Am J Physiol Renal Physiol. 2009. PMID: 19261745 Free PMC article.
-
Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury.Am J Physiol Renal Physiol. 2010 Aug;299(2):F426-35. doi: 10.1152/ajprenal.00248.2010. Epub 2010 May 26. Am J Physiol Renal Physiol. 2010. PMID: 20504881 Free PMC article.
-
Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure.J Am Soc Nephrol. 2008 Jul;19(7):1321-30. doi: 10.1681/ASN.2007121368. Epub 2008 Apr 16. J Am Soc Nephrol. 2008. PMID: 18417719 Free PMC article.
-
Heme Burden and Ensuing Mechanisms That Protect the Kidney: Insights from Bench and Bedside.Int J Mol Sci. 2021 Jul 30;22(15):8174. doi: 10.3390/ijms22158174. Int J Mol Sci. 2021. PMID: 34360940 Free PMC article. Review.
-
Proximal Tubular Transcription Factors in Acute Kidney Injury: Recent Advances.Nephron. 2020;144(12):613-615. doi: 10.1159/000508856. Epub 2020 Jul 9. Nephron. 2020. PMID: 32645697 Free PMC article. Review.
Cited by
-
Heme Proteins and Kidney Injury: Beyond Rhabdomyolysis.Kidney360. 2022 Oct 5;3(11):1969-1979. doi: 10.34067/KID.0005442022. eCollection 2022 Nov 24. Kidney360. 2022. PMID: 36514409 Free PMC article. Review.
-
Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury.Am J Physiol Renal Physiol. 2011 Mar;300(3):F628-38. doi: 10.1152/ajprenal.00654.2010. Epub 2010 Dec 8. Am J Physiol Renal Physiol. 2011. PMID: 21147844 Free PMC article.
-
The roles of heme oxygenase-1 in renal disease.Front Nephrol. 2023 May 5;3:1156346. doi: 10.3389/fneph.2023.1156346. eCollection 2023. Front Nephrol. 2023. PMID: 37675385 Free PMC article. Review.
-
New insights into the role of heme oxygenase-1 in acute kidney injury.Kidney Res Clin Pract. 2020 Dec 31;39(4):387-401. doi: 10.23876/j.krcp.20.091. Kidney Res Clin Pract. 2020. PMID: 33184238 Free PMC article. Review.
-
Role of TLR4 signaling in the nephrotoxicity of heme and heme proteins.Am J Physiol Renal Physiol. 2018 May 1;314(5):F906-F914. doi: 10.1152/ajprenal.00432.2017. Epub 2017 Oct 4. Am J Physiol Renal Physiol. 2018. PMID: 28978536 Free PMC article.
References
-
- Baliga R, Zhang Z, Baliga M, Ueda N, Shas SV. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int 54: 15623–1569, 1998 - PubMed
-
- Baliga R, Zhang M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996 - PubMed
-
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, 3rd, Gingeras TR. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120: 169–181, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

