Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice
- PMID: 20032118
- PMCID: PMC2838591
- DOI: 10.1152/ajprenal.00439.2009
Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice
Erratum in
- Am J Physiol Renal Physiol. 2014 Jun 1;306(11):F1392. Jia, Zhunjun [corrected to Jia, Zhanjun]
Abstract
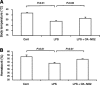
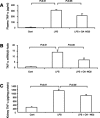
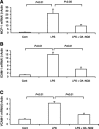
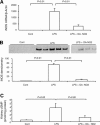
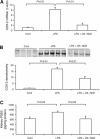
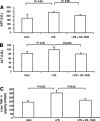
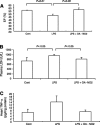
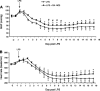
Nitroalkene derivatives of nitro-oleic acid (OA-NO2) are endogenous lipid products with potent anti-inflammatory properties in vitro. The present study was undertaken to evaluate the in vivo anti-inflammatory effect of OA-NO2 in mice given LPS. Two days before LPS administration, C57BL/6J mice were chronically infused with vehicle (LPS vehicle) or OA-NO2 (LPS OA-NO2) at 200 microg x kg(-1) x day(-1) via osmotic minipumps; LPS was administered via a single intraperitoneal (ip) injection (10 mg/kg in saline). A third group received an ip injection of saline without LPS or OA-NO2 and served as controls. At 18 h of LPS administration, LPS vehicle mice displayed multiorgan dysfunction as evidenced by elevated plasma urea and creatinine (kidney), aspartate aminotransferase (AST) and alanine aminotransferase (ALT; liver), and lactate dehydrogenase (LDH) and reduced ejection fraction (heart). In contrast, the severity of multiorgan dysfunction was less in LPS OA-NO2 animals. The levels of circulating TNF-alpha and renal TNF-alpha mRNA expression, together with renal mRNA expression of monocyte chemoattractant protein-1, ICAM-1, and VCAM-1, and with renal mRNA and protein expression of inducible nitric oxide synthase and cyclooxygenase 2, and renal cGMP and PGE2 contents, were greater in LPS vehicle vs. control mice, but were attenuated in LPS OA-NO2 animals. Similar patterns of changes in the expression of inflammatory mediators were observed in the liver. Together, pretreatment with OA-NO2 ameliorated the inflammatory response and multiorgan injury in endotoxin-induced endotoxemia in mice.
Figures


Similar articles
-
Nitrooleic acid protects against cisplatin nephropathy: role of COX-2/mPGES-1/PGE2 cascade.Mediators Inflamm. 2015;2015:293474. doi: 10.1155/2015/293474. Epub 2015 Mar 11. Mediators Inflamm. 2015. PMID: 25861160 Free PMC article.
-
Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury.Am J Physiol Renal Physiol. 2008 Oct;295(4):F942-9. doi: 10.1152/ajprenal.90236.2008. Epub 2008 Aug 27. Am J Physiol Renal Physiol. 2008. PMID: 18753300 Free PMC article.
-
Pretreatment with Rho-kinase inhibitor ameliorates lethal endotoxemia-induced liver injury by improving mitochondrial function.Int Immunopharmacol. 2016 Nov;40:125-130. doi: 10.1016/j.intimp.2016.08.036. Epub 2016 Aug 30. Int Immunopharmacol. 2016. PMID: 27588912
-
The effect of angiotensin receptor blockers on C-reactive protein and other circulating inflammatory indices in man.Vasc Health Risk Manag. 2009;5(1):233-42. doi: 10.2147/vhrm.s4800. Epub 2009 Apr 8. Vasc Health Risk Manag. 2009. PMID: 19436669 Free PMC article. Review.
-
Nitrolipids in kidney physiology and disease.Nitric Oxide. 2018 Mar 29:S1089-8603(18)30006-5. doi: 10.1016/j.niox.2018.03.021. Online ahead of print. Nitric Oxide. 2018. PMID: 29605557 Free PMC article. Review.
Cited by
-
Nitro-oleic acid is a novel anti-oxidative therapy for diabetic kidney disease.Am J Physiol Renal Physiol. 2013 Dec 1;305(11):F1542-3. doi: 10.1152/ajprenal.00489.2013. Epub 2013 Sep 18. Am J Physiol Renal Physiol. 2013. PMID: 24049149 Free PMC article. No abstract available.
-
Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts.Cardiovasc Res. 2013 Apr 1;98(1):116-24. doi: 10.1093/cvr/cvt002. Epub 2013 Jan 17. Cardiovasc Res. 2013. PMID: 23334216 Free PMC article.
-
The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid.J Biol Chem. 2017 Jan 27;292(4):1145-1159. doi: 10.1074/jbc.M116.756288. Epub 2016 Dec 6. J Biol Chem. 2017. PMID: 27923813 Free PMC article.
-
Evaluation of 10-Nitro Oleic Acid Bio-Elimination in Rats and Humans.Sci Rep. 2017 Jan 5;7:39900. doi: 10.1038/srep39900. Sci Rep. 2017. PMID: 28054588 Free PMC article.
-
Nitrooleic acid protects against cisplatin nephropathy: role of COX-2/mPGES-1/PGE2 cascade.Mediators Inflamm. 2015;2015:293474. doi: 10.1155/2015/293474. Epub 2015 Mar 11. Mediators Inflamm. 2015. PMID: 25861160 Free PMC article.
References
-
- Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1137–L1145, 2000 - PubMed
-
- Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, Kudsk K, Bruining HA, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial Ro 45–2081 Study Group. JAMA 277: 1531–1538, 1997 - PubMed
-
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280: 42464–42475, 2005 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

