Expression of transporters involved in urine concentration recovers differently after cessation of lithium treatment
- PMID: 20032119
- PMCID: PMC2838592
- DOI: 10.1152/ajprenal.00424.2009
Expression of transporters involved in urine concentration recovers differently after cessation of lithium treatment
Abstract
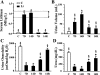
Patients receiving lithium therapy, an effective treatment for bipolar disorder, often present with acquired nephrogenic diabetes insipidus. The nephrotoxic effects of lithium can be detected 3 wk after the start of treatment and many of these symptoms may disappear in a few weeks after lithium use is stopped. Most patients, however, still have a urine-concentrating defect years after ending treatment. This prompted an investigation of the transporters involved in the urine concentration mechanism, UT-A1, UT-A3, aquaporin-2 (AQP2), and NKCC2, after discontinuing lithium therapy. Sprague-Dawley rats fed a Li2CO3-supplemented diet produced large volumes of dilute urine after 14 days. After lithium treatment was discontinued, urine osmolality returned to normal within 14 days but urine volume and urine urea failed to reach basal levels. Western blot and immunohistochemical analyses revealed that both urea transporters UT-A1 and UT-A3 were reduced at 7 and 14 days of lithium treatment and both transporters recovered to basal levels 14 days after discontinuing lithium administration. Similar analyses demonstrated a decrease in AQP2 expression after 7 and 14 days of lithium therapy. AQP2 expression increased over the 7 and 14 days following the cessation of lithium but failed to recover to normal levels. NKCC2 expression was unaltered during the 14-day lithium regimen but did increase 14 days after the treatment was stopped. In summary, the rapid restoration of UT-A1 and UT-A3 as well as the increased expression of NKCC2 are critical components to the reestablishment of urine concentration after lithium treatment.
Figures
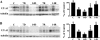

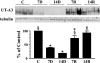


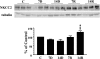

References
-
- Anai H, Ueta Y, Serino R, Nomura M, Kabashima N, Shibuya I, Takasugi M, Nakashima Y, Yamashita H. Upregulation of the expression of vasopressin gene in the paraventricular and supraoptic nuclei of the lithium-induced diabetes insipidus rat. Brain Res 772: 161–166, 1997 - PubMed
-
- Bedford JJ, Leader JP, Jing R, Walker LJ, Klein JD, Sands JM, Walker RJ. Amiloride restores renal medullary osmolytes in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 294: F812–F820, 2008 - PubMed
-
- Bichet DG. Nephrogenic diabetes insipidus. Am J Med 105: 431–442, 1998 - PubMed
-
- Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

