Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666
- PMID: 20032143
- PMCID: PMC2811274
- DOI: 10.1148/radiol.2541090953
Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666
Abstract
Purpose: To determine reasons for nonparticipation in a trial of supplemental screening with magnetic resonance (MR) imaging after mammography and ultrasonography (US).
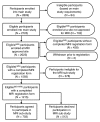
Materials and methods: Women(n = 2809) at elevated risk of breast cancer were enrolled in the American College of Radiology Imaging Network 6666 US Screening Protocol at 21 institutions. Fourteen institutions met technical and experience requirements for this institutional review board-approved, HIPAA-compliant substudy of supplemental screening with MR imaging. Those women who had completed 0-, 12-, and 24-month screenings with mammography combined with US were considered for a single contrast material-enhanced MR examination within 8 weeks after completing the 24-month mammography-US screening. A total of 1593 women had complete MR substudy registration data: 378 of them were ineligible for the study, and 1215 had analyzable data. Reasons for nonparticipation were determined. Demographic data were compared between study participants and nonparticipants.
Results: Of 1215 women with analyzable data, 703 (57.9%), with a mean age of 54.8 years, were enrolled in the MR substudy and 512 (42.1%) declined participation. Women with a 25% or greater lifetime risk of breast cancer were more likely to participate (odds ratio, 1.53; 95% confidence interval: 1.10, 2.12). Of 512 nonparticipants, 130 (25.4%) refused owing to claustrophobia; 93 (18.2%), owing to time constraints; 62 (12.1%), owing to financial concerns; 47 (9.2%), because their physician would not provide a referral and/or did not believe MR imaging was indicated; 40 (7.8%), because they were not interested; 39 (7.6%), because they were medically intolerant to MR imaging; 29 (5.7%), because they did not want to undergo intravenous injection; 27 (5.3%), owing to additional biopsy or other procedures that might be required subsequently; 21 (4.1%), owing to MR imaging scheduling constraints; 11 (2.2%), because of the travel required; seven (1.4%), owing to gadolinium-related risks or allergies; and six (1.2%), for unknown reasons.
Conclusion: Of 1215 women with elevated breast cancer risk who could, according to protocol guidelines, undergo breast MR imaging, only 57.9% agreed to participate.
Figures
Comment in
-
Claustrophobia preventing MR imaging of the breast.Radiology. 2010 Jul;256(1):328; author reply 328-9. doi: 10.1148/radiol.100113. Radiology. 2010. PMID: 20574107 No abstract available.
References
-
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009;192:390–399 - PubMed
-
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92:1081–1087 - PubMed
-
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89 - PubMed
-
- Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001;19:924–930 - PubMed
-
- Berg WA. Rationale for a trial of screening breast ultrasound: American College of Radiology Imaging Network (ACRIN) 6666. AJR Am J Roentgenol 2003;180:1225–1228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous