Herpes simplex virus type 1 immediate-early protein ICP22 is required for VICE domain formation during productive viral infection
- PMID: 20032172
- PMCID: PMC2820935
- DOI: 10.1128/JVI.01686-09
Herpes simplex virus type 1 immediate-early protein ICP22 is required for VICE domain formation during productive viral infection
Abstract
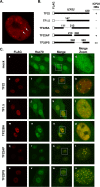
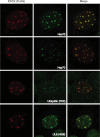
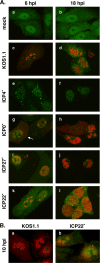
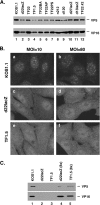
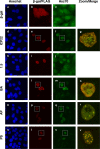
During productive infection, herpes simplex virus type 1 (HSV-1) induces the formation of discrete nuclear foci containing cellular chaperone proteins, proteasomal components, and ubiquitinated proteins. These structures are known as VICE domains and are hypothesized to play an important role in protein turnover and nuclear remodeling in HSV-1-infected cells. Here we show that VICE domain formation in Vero and other cells requires the HSV-1 immediate-early protein ICP22. Since ICP22 null mutants replicate efficiently in Vero cells despite being unable to induce VICE domain formation, it can be concluded that VICE domain formation is not essential for HSV-1 productive infection. However, our findings do not exclude the possibility that VICE domain formation is required for viral replication in cells that are nonpermissive for ICP22 mutants. Our studies also show that ICP22 itself localizes to VICE domains, suggesting that it could play a role in forming these structures. Consistent with this, we found that ICP22 expression in transfected cells is sufficient to reorganize the VICE domain component Hsc70 into nuclear inclusion bodies that resemble VICE domains. An N-terminal segment of ICP22, corresponding to residues 1 to 146, is critical for VICE domain formation in infected cells and Hsc70 reorganization in transfected cells. We previously found that this portion of the protein is dispensable for ICP22's effects on RNA polymerase II phosphorylation. Thus, ICP22 mediates two distinct regulatory activities that both modify important components of the host cell nucleus.
Figures


References
-
- Besse, S., and F. Puvion-Dutilleul. 1996. Intranuclear retention of ribosomal RNAs in response to herpes simplex virus type 1 infection. J. Cell Sci. 109:119-129. - PubMed
-
- Brandt, C. R., A. W. Kolb, D. D. Shah, A. M. Pumfery, R. L. Kintner, E. Jaehnig, and J. J. Van Gompel. 2003. Multiple determinants contribute to the virulence of HSV ocular and CNS infection and identification of serine 34 of the US1 gene as an ocular disease determinant. Invest. Ophthalmol. Vis. Sci. 44:2657-2668. - PubMed
-
- Bukau, B., J. Weissman, and A. Horwich. 2006. Molecular chaperones and protein quality control. Cell 125:443-451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

