Vesicular stomatitis virus genomic RNA persists in vivo in the absence of viral replication
- PMID: 20032173
- PMCID: PMC2838132
- DOI: 10.1128/JVI.02052-09
Vesicular stomatitis virus genomic RNA persists in vivo in the absence of viral replication
Abstract
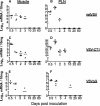
Our previous studies using intranasal inoculation of mice with vesicular stomatitis virus (VSV) vaccine vectors showed persistence of vector genomic RNA (gRNA) for at least 60 days in lymph nodes in the absence of detectable infectious virus. Here we show high-level concentration of virus and gRNA in lymph nodes after intramuscular inoculation of mice with attenuated or single-cycle VSV vectors as well as long-term persistence of gRNA in the lymph nodes. To determine if the persistence of gRNA was due to ongoing viral replication, we developed a tagged-primer approach that was critical for detection of VSV mRNA specifically. Our results show that VSV gRNA persists long-term in the lymph nodes while VSV mRNA is present only transiently. Because VSV transcription is required for replication, our results indicate that persistence of gRNA does not result from continuing viral replication. We also performed macrophage depletion studies that are consistent with initial trapping of VSV gRNA largely in lymph node macrophages and subsequent persistence elsewhere in the lymph node.
Figures



Similar articles
-
Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA.J Virol. 2007 Feb;81(4):2078-82. doi: 10.1128/JVI.02525-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151110 Free PMC article.
-
Homotypic and heterotypic exclusion of vesicular stomatitis virus replication by high levels of recombinant polymerase protein L.J Virol. 1987 Oct;61(10):3133-42. doi: 10.1128/JVI.61.10.3133-3142.1987. J Virol. 1987. PMID: 3041035 Free PMC article.
-
Experimental vesicular stomatitis virus infection of swine: extent of infection and immunological response.Vet Immunol Immunopathol. 1989 Mar;20(4):345-61. doi: 10.1016/0165-2427(89)90080-9. Vet Immunol Immunopathol. 1989. PMID: 2470190
-
Defective interfering virus particles modulate virulence.J Virol. 1985 Aug;55(2):366-73. doi: 10.1128/JVI.55.2.366-373.1985. J Virol. 1985. PMID: 2991562 Free PMC article.
-
Experimental Evolution Generates Novel Oncolytic Vesicular Stomatitis Viruses with Improved Replication in Virus-Resistant Pancreatic Cancer Cells.J Virol. 2020 Jan 17;94(3):e01643-19. doi: 10.1128/JVI.01643-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694943 Free PMC article.
Cited by
-
Field evaluation of a Pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak.Sci Rep. 2020 May 26;10(1):8724. doi: 10.1038/s41598-020-65736-0. Sci Rep. 2020. PMID: 32457420 Free PMC article.
-
The Propagation, Quantification, and Storage of Vesicular Stomatitis Virus.Curr Protoc Microbiol. 2020 Sep;58(1):e110. doi: 10.1002/cpmc.110. Curr Protoc Microbiol. 2020. PMID: 32833351 Free PMC article.
-
A Single Amino Acid Substitution in the Matrix Protein (M51R) of Vesicular Stomatitis New Jersey Virus Impairs Replication in Cultured Porcine Macrophages and Results in Significant Attenuation in Pigs.Front Microbiol. 2020 May 29;11:1123. doi: 10.3389/fmicb.2020.01123. eCollection 2020. Front Microbiol. 2020. PMID: 32587580 Free PMC article.
-
Monocytes and Macrophages as Viral Targets and Reservoirs.Int J Mol Sci. 2018 Sep 18;19(9):2821. doi: 10.3390/ijms19092821. Int J Mol Sci. 2018. PMID: 30231586 Free PMC article. Review.
-
Agonistic anti-CD40 enhances the CD8+ T cell response during vesicular stomatitis virus infection.PLoS One. 2014 Aug 28;9(8):e106060. doi: 10.1371/journal.pone.0106060. eCollection 2014. PLoS One. 2014. PMID: 25166494 Free PMC article.
References
-
- Barrera, J. C., and G. J. Letchworth. 1996. Persistence of vesicular stomatitis virus New Jersey RNA in convalescent hamsters. Virology 219:453-464. - PubMed
-
- Ciavarra, R. P., K. Buhrer, N. Van Rooijen, and B. Tedeschi. 1997. T cell priming against vesicular stomatitis virus analyzed in situ: red pulp macrophages, but neither marginal metallophilic nor marginal zone macrophages, are required for priming CD4+ and CD8+ T cells. J. Immunol. 158:1749-1755. - PubMed
-
- Clarke, D. K., F. Nasar, M. Lee, J. E. Johnson, K. Wright, P. Calderon, M. Guo, R. Natuk, D. Cooper, R. M. Hendry, and S. A. Udem. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056-2064. - PMC - PubMed
-
- Connolly, J. H., I. V. Allen, L. J. Hurwitz, and J. H. Millar. 1967. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet i:542-544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

