Multi-low-dose mucosal simian immunodeficiency virus SIVmac239 challenge of cynomolgus macaques immunized with "hyperattenuated" SIV constructs
- PMID: 20032177
- PMCID: PMC2820907
- DOI: 10.1128/JVI.01995-09
Multi-low-dose mucosal simian immunodeficiency virus SIVmac239 challenge of cynomolgus macaques immunized with "hyperattenuated" SIV constructs
Abstract
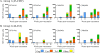
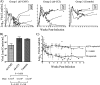
Hyperattenuated simian immunodeficiency virus SIVmac239-derived constructs Delta5-CMV and Delta6-CCI are an effort to render SIV incapable of, in practical terms, both reversion and recombination while maintaining the immune features of SIV as a retrovirus. Primary inoculation of cynomolgus macaques with 10(8) 50% tissue culture infective doses (TCID(50)) of Delta5-CMV or Delta6-CCI induced low-level humoral and cellular responses detectable in the absence of measureable in vivo replication. The first of three DNA boosts resulted in elevated gamma interferon (IFN-gamma) enzyme-linked immunospot (ELISPOT) responses to Gag, Pol, and Env in the Delta5-CMV vaccine group compared to the Delta6-CCI vaccine group (P = 0.001). Weekly intrarectal challenge with a low dose of SIVmac239 followed by a dose escalation was conducted until all animals became infected. The mean peak viral load of the Delta5-CMV-vaccinated animals (3.7 x 10(5) copies/ml) was approximately 1 log unit lower than that of the control animals. More dramatically, the viral load set point of these animals was decreased by 3 log units compared to that of the controls (<50 versus 1.64 x 10(4) copies/ml; P < 0.0001). Seventy-five percent (6/8) of vaccine recipients controlled virus below 1,000 copies/ml for at least 6 months, with a subset controlling virus and maintaining substantial CD4 T-cell counts for close to 2 years of follow-up. The correlates of protection from SIV disease progression may lie in the rapidity and protective value of immune responses that occur early in primary SIV infection. Prior immunization with hyperattenuated SIVmac239, even if sterilizing immunity is not achieved, may allow a more advantageous host response.
Figures



Similar articles
-
Vaccination of Macaques with DNA Followed by Adenoviral Vectors Encoding Simian Immunodeficiency Virus (SIV) Gag Alone Delays Infection by Repeated Mucosal Challenge with SIV.J Virol. 2019 Oct 15;93(21):e00606-19. doi: 10.1128/JVI.00606-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413132 Free PMC article.
-
T-cell-mediated protective efficacy of a systemic vaccine approach in cynomolgus monkeys after SIV mucosal challenge.J Med Primatol. 2004 Oct;33(5-6):251-61. doi: 10.1111/j.1600-0684.2004.00076.x. J Med Primatol. 2004. PMID: 15525326
-
Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen.J Virol. 2011 Sep;85(18):9578-87. doi: 10.1128/JVI.05060-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734035 Free PMC article.
-
Rectal Acquisition of Simian Immunodeficiency Virus (SIV) SIVmac239 Infection despite Vaccine-Induced Immune Responses against the Entire SIV Proteome.J Virol. 2020 Nov 23;94(24):e00979-20. doi: 10.1128/JVI.00979-20. Print 2020 Nov 23. J Virol. 2020. PMID: 33028714 Free PMC article.
-
Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus.J Virol. 2011 Dec;85(23):12708-20. doi: 10.1128/JVI.00865-11. Epub 2011 Sep 7. J Virol. 2011. PMID: 21900170 Free PMC article.
Cited by
-
Vaccine targeting SIVmac251 protease cleavage sites protects macaques against vaginal infection.J Clin Invest. 2020 Dec 1;130(12):6429-6442. doi: 10.1172/JCI138728. J Clin Invest. 2020. PMID: 32853182 Free PMC article.
-
Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV.Immunol Rev. 2011 Jan;239(1):109-24. doi: 10.1111/j.1600-065X.2010.00968.x. Immunol Rev. 2011. PMID: 21198668 Free PMC article. Review.
-
Study of MHC class II region polymorphism in the Filipino cynomolgus macaque population.Immunogenetics. 2014 Apr;66(4):219-30. doi: 10.1007/s00251-014-0764-7. Epub 2014 Feb 26. Immunogenetics. 2014. PMID: 24569954
-
Correlation between antidrug antibodies, pre-existing antidrug reactivity, and immunogenetics (MHC class II alleles) in cynomolgus macaque.Immunogenetics. 2019 Nov;71(10):605-615. doi: 10.1007/s00251-019-01136-7. Epub 2019 Nov 27. Immunogenetics. 2019. PMID: 31776588
-
Immunologic and Virologic Mechanisms for Partial Protection from Intravenous Challenge by an Integration-Defective SIV Vaccine.Viruses. 2017 Jun 2;9(6):135. doi: 10.3390/v9060135. Viruses. 2017. PMID: 28574482 Free PMC article.
References
-
- Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A*01: implications for vaccine design and testing. J. Virol. 75:738-749. - PMC - PubMed
-
- Almond, N., K. Kent, M. Cranage, E. Rud, B. Clarke, and E. J. Stott. 1995. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet 345:1342-1344. - PubMed
-
- Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. - PubMed
-
- Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

