The interferon stimulator mitochondrial antiviral signaling protein facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases
- PMID: 20032188
- PMCID: PMC2820939
- DOI: 10.1128/JVI.02174-09
The interferon stimulator mitochondrial antiviral signaling protein facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases
Abstract
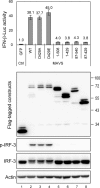
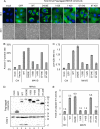
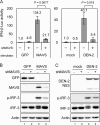
Interferon (IFN) signaling is initiated by the recognition of viral components by host pattern recognition receptors. Dengue virus (DEN) triggers IFN-beta induction through a molecular mechanism involving the cellular RIG-I/MAVS signaling pathway. Here we report that the MAVS protein level is reduced in DEN-infected cells and that caspase-1 and caspase-3 cleave MAVS at residue D429. In addition to its well-known function in IFN induction, MAVS is also a proapoptotic molecule that triggers disruption of the mitochondrial membrane potential and activation of caspases. Although different domains are required for the induction of cytotoxicity and IFN, caspase cleavage at residue 429 abolished both functions of MAVS. The apoptotic role of MAVS in viral infection and double-stranded RNA (dsRNA) stimulation was demonstrated in cells with reduced endogenous MAVS expression induced by RNA interference. Even though IFN-beta promoter activation was largely suppressed, DEN production was not affected greatly in MAVS knockdown cells. Instead, DEN- and dsRNA-induced cell death and caspase activation were delayed and attenuated in the cells with reduced levels of MAVS. These results reveal a new role of MAVS in the regulation of cell death beyond its well-known function of IFN induction in antiviral innate immunity.
Figures







Similar articles
-
MAVS-dependent IRF3/7 bypass of interferon β-induction restricts the response to measles infection in CD150Tg mouse bone marrow-derived dendritic cells.Mol Immunol. 2014 Feb;57(2):100-10. doi: 10.1016/j.molimm.2013.08.007. Epub 2013 Oct 4. Mol Immunol. 2014. PMID: 24096085
-
How Dengue Virus Circumvents Innate Immunity.Front Immunol. 2018 Dec 4;9:2860. doi: 10.3389/fimmu.2018.02860. eCollection 2018. Front Immunol. 2018. PMID: 30564245 Free PMC article. Review.
-
Dengue Virus Subverts Host Innate Immunity by Targeting Adaptor Protein MAVS.J Virol. 2016 Jul 27;90(16):7219-7230. doi: 10.1128/JVI.00221-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252539 Free PMC article.
-
DHX15 and Rig-I Coordinate Apoptosis and Innate Immune Signaling by Antiviral RNase L.Viruses. 2024 Dec 13;16(12):1913. doi: 10.3390/v16121913. Viruses. 2024. PMID: 39772220 Free PMC article.
-
Regulation of MAVS Expression and Signaling Function in the Antiviral Innate Immune Response.Front Immunol. 2020 May 27;11:1030. doi: 10.3389/fimmu.2020.01030. eCollection 2020. Front Immunol. 2020. PMID: 32536927 Free PMC article. Review.
Cited by
-
A long-awaited merger of the pathways mediating host defence and programmed cell death.Nat Rev Immunol. 2014 Sep;14(9):601-18. doi: 10.1038/nri3720. Nat Rev Immunol. 2014. PMID: 25145756 Review.
-
Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice.Hepatology. 2011 Jun;53(6):1917-31. doi: 10.1002/hep.24301. Epub 2011 May 2. Hepatology. 2011. PMID: 21425308 Free PMC article.
-
TAX1BP1 Restrains Virus-Induced Apoptosis by Facilitating Itch-Mediated Degradation of the Mitochondrial Adaptor MAVS.Mol Cell Biol. 2016 Dec 19;37(1):e00422-16. doi: 10.1128/MCB.00422-16. Print 2017 Jan 1. Mol Cell Biol. 2016. PMID: 27736772 Free PMC article.
-
Interplay between Inflammation and Cellular Stress Triggered by Flaviviridae Viruses.Front Microbiol. 2016 Aug 25;7:1233. doi: 10.3389/fmicb.2016.01233. eCollection 2016. Front Microbiol. 2016. PMID: 27610098 Free PMC article. Review.
-
Dengue Virus Impairs Mitochondrial Fusion by Cleaving Mitofusins.PLoS Pathog. 2015 Dec 30;11(12):e1005350. doi: 10.1371/journal.ppat.1005350. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26717518 Free PMC article.
References
-
- Besch, R., H. Poeck, T. Hohenauer, D. Senft, G. Hacker, C. Berking, V. Hornung, S. Endres, T. Ruzicka, S. Rothenfusser, and G. Hartmann. 2009. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J. Clin. Invest. 119:2399-2411. - PMC - PubMed
-
- Bhoj, V. G., Q. Sun, E. J. Bhoj, C. Somers, X. Chen, J. P. Torres, A. Mejias, A. M. Gomez, H. Jafri, O. Ramilo, and Z. J. Chen. 2008. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 105:14046-14051. - PMC - PubMed
-
- Chang, T. H., C. L. Liao, and Y. L. Lin. 2006. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect. 8:157-171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

