Conserved motifs within Ebola and Marburg virus VP40 proteins are important for stability, localization, and subsequent budding of virus-like particles
- PMID: 20032189
- PMCID: PMC2820906
- DOI: 10.1128/JVI.02034-09
Conserved motifs within Ebola and Marburg virus VP40 proteins are important for stability, localization, and subsequent budding of virus-like particles
Abstract
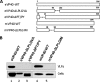
The filovirus VP40 protein is capable of budding from mammalian cells in the form of virus-like particles (VLPs) that are morphologically indistinguishable from infectious virions. Ebola virus VP40 (eVP40) contains well-characterized overlapping L domains, which play a key role in mediating efficient virus egress. L domains represent only one component required for efficient budding and, therefore, there is a need to identify and characterize additional domains important for VP40 function. We demonstrate here that the (96)LPLGVA(101) sequence of eVP40 and the corresponding (84)LPLGIM(89) sequence of Marburg virus VP40 (mVP40) are critical for efficient release of VP40 VLPs. Indeed, deletion of these motifs essentially abolished the ability of eVP40 and mVP40 to bud as VLPs. To address the mechanism by which the (96)LPLGVA(101) motif of eVP40 contributes to egress, a series of point mutations were introduced into this motif. These mutants were then compared to the eVP40 wild type in a VLP budding assay to assess budding competency. Confocal microscopy and gel filtration analyses were performed to assess their pattern of intracellular localization and ability to oligomerize, respectively. Our results show that mutations disrupting the (96)LPLGVA(101) motif resulted in both altered patterns of intracellular localization and self-assembly compared to wild-type controls. Interestingly, coexpression of either Ebola virus GP-WT or mVP40-WT with eVP40-DeltaLPLGVA failed to rescue the budding defective eVP40-DeltaLPLGVA mutant into VLPs; however, coexpression of eVP40-WT with mVP40-DeltaLPLGIM successfully rescued budding of mVP40-DeltaLPLGIM into VLPs at mVP40-WT levels. In sum, our findings implicate the LPLGVA and LPLGIM motifs of eVP40 and mVP40, respectively, as being important for VP40 structure/stability and budding.
Figures


Similar articles
-
Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress.J Virol. 2017 Sep 27;91(20):e00812-17. doi: 10.1128/JVI.00812-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768865 Free PMC article.
-
Ubiquitin Ligase SMURF2 Interacts with Filovirus VP40 and Promotes Egress of VP40 VLPs.Viruses. 2021 Feb 12;13(2):288. doi: 10.3390/v13020288. Viruses. 2021. PMID: 33673144 Free PMC article.
-
ITCH E3 Ubiquitin Ligase Interacts with Ebola Virus VP40 To Regulate Budding.J Virol. 2016 Sep 29;90(20):9163-71. doi: 10.1128/JVI.01078-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489272 Free PMC article.
-
Host and Viral Proteins Modulating Ebola and Marburg Virus Egress.Viruses. 2019 Jan 3;11(1):25. doi: 10.3390/v11010025. Viruses. 2019. PMID: 30609802 Free PMC article. Review.
-
Conformational plasticity of the Ebola virus matrix protein.Protein Sci. 2014 Nov;23(11):1519-27. doi: 10.1002/pro.2541. Epub 2014 Sep 4. Protein Sci. 2014. PMID: 25159197 Free PMC article. Review.
Cited by
-
The multifunctional Ebola virus VP40 matrix protein is a promising therapeutic target.Future Virol. 2015 May;10(5):537-546. doi: 10.2217/fvl.15.6. Future Virol. 2015. PMID: 26120351 Free PMC article.
-
Alphavirus Replicon DNA Vectors Expressing Ebola GP and VP40 Antigens Induce Humoral and Cellular Immune Responses in Mice.Front Microbiol. 2018 Jan 10;8:2662. doi: 10.3389/fmicb.2017.02662. eCollection 2017. Front Microbiol. 2018. PMID: 29375526 Free PMC article.
-
Small-molecule probes targeting the viral PPxY-host Nedd4 interface block egress of a broad range of RNA viruses.J Virol. 2014 Jul;88(13):7294-306. doi: 10.1128/JVI.00591-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741084 Free PMC article.
-
Lipid-specific oligomerization of the Marburg virus matrix protein VP40 is regulated by two distinct interfaces for virion assembly.J Biol Chem. 2021 Jan-Jun;296:100796. doi: 10.1016/j.jbc.2021.100796. Epub 2021 May 18. J Biol Chem. 2021. PMID: 34019871 Free PMC article.
-
Angiomotin Counteracts the Negative Regulatory Effect of Host WWOX on Viral PPxY-Mediated Egress.J Virol. 2021 Mar 25;95(8):e00121-21. doi: 10.1128/JVI.00121-21. Epub 2021 Feb 3. J Virol. 2021. PMID: 33536174 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

