Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions
- PMID: 20032193
- PMCID: PMC2820934
- DOI: 10.1128/JVI.02256-09
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions
Abstract
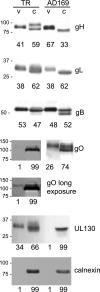
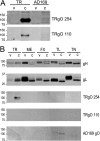
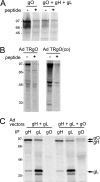
Human cytomegalovirus (HCMV) produces the following two gH/gL complexes: gH/gL/gO and gH/gL/UL128-131. Entry into epithelial and endothelial cells requires gH/gL/UL128-131, and we have provided evidence that gH/gL/UL128-131 binds saturable epithelial cell receptors to mediate entry. HCMV does not require gH/gL/UL128-131 to enter fibroblasts, and laboratory adaptation to fibroblasts results in mutations in the UL128-131 genes, abolishing infection of epithelial and endothelial cells. HCMV gO-null mutants produce very small plaques on fibroblasts yet can spread on endothelial cells. Thus, one prevailing model suggests that gH/gL/gO mediates infection of fibroblasts, while gH/gL/UL128-131 mediates entry into epithelial/endothelial cells. Most biochemical studies of gO have involved the HCMV lab strain AD169, which does not assemble gH/gL/UL128-131 complexes. We examined gO produced by the low-passage clinical HCMV strain TR. Surprisingly, TR gO was not detected in purified extracellular virus particles. In TR-infected cells, gO remained sensitive to endoglycosidase H, suggesting that the protein was not exported from the endoplasmic reticulum (ER). However, TR gO interacted with gH/gL in the ER and promoted export of gH/gL from the ER to the Golgi apparatus. Pulse-chase experiments showed that a fraction of gO remained bound to gH/gL for relatively long periods, but gO eventually dissociated or was degraded and was not found in extracellular virions or secreted from cells. The accompanying report by P. T. Wille et al. (J. Virol., 84:2585-2596, 2010) showed that a TR gO-null mutant failed to incorporate gH/gL into virions and that the mutant was unable to enter fibroblasts and epithelial and endothelial cells. We concluded that gO acts as a molecular chaperone, increasing gH/gL ER export and incorporation into virions. It appears that gO competes with UL128-131 for binding onto gH/gL but is released from gH/gL, so that gH/gL (lacking UL128-131) is incorporated into virions. Thus, our revised model suggests that both gH/gL and gH/gL/UL128-131 are required for entry into epithelial and endothelial cells.
Figures






References
-
- Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87:2451-2460. - PubMed
-
- Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84:1117-1122. - PubMed
-
- Bogner, E., M. Reschke, B. Reis, E. Reis, W. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 126:67-80. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
-
- Britt, W. J., and C. A. Alford. 1996. Cytomegaloviruses, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Press, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

