Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation
- PMID: 20032195
- PMCID: PMC2817602
- DOI: 10.1210/me.2009-0346
Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation
Abstract
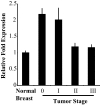
The current statistics associated with breast cancer continue to show a relatively high recurrence rate together with a poor survival for aggressive metastatic disease. These findings reflect, in part, the pharmaceutical intractability of processes involved in the metastatic process and highlight the need to identify additional drug targets for the treatment of late-stage disease. In the current study, we report that ligand activation of the aryl-hydrocarbon receptor (AhR) inhibits multiple aspects of the metastatic process in a panel of breast cancer cell lines that represent the major breast cancer subtypes. Specifically, it was observed that treatment with exogenous AhR agonists significantly inhibited cell invasiveness and motility in the Boyden chamber assay and inhibited colony formation in soft agar regardless of estrogen receptor (ER), progesterone receptor, or human epidermal growth factor receptor 2 status. Knockdown of the AhR using small interfering RNA duplexes demonstrated that the inhibition of invasiveness was receptor dependent and that endogenous receptor activity was protective in each cell type examined. The inhibition of invasiveness and anchorage-independent growth correlated with the ability of exogenous AhR agonists to promote differentiation. Finally, exogenous AhR agonists were able to promote differentiation in a putative mammary cancer stem cell line. Cumulatively, these results suggest that the AhR plays an important role in mammary epithelial differentiation and, as such, represent a promising therapeutic target for a range of phenotypically distinct human breast cancers.
Figures



Similar articles
-
Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist.PLoS One. 2010 Nov 3;5(11):e13831. doi: 10.1371/journal.pone.0013831. PLoS One. 2010. PMID: 21072210 Free PMC article.
-
Towards Resolving the Pro- and Anti-Tumor Effects of the Aryl Hydrocarbon Receptor.Int J Mol Sci. 2018 May 7;19(5):1388. doi: 10.3390/ijms19051388. Int J Mol Sci. 2018. PMID: 29735912 Free PMC article.
-
The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells.BMC Biol. 2016 Mar 16;14:20. doi: 10.1186/s12915-016-0240-y. BMC Biol. 2016. PMID: 26984638 Free PMC article.
-
Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells.J Mammary Gland Biol Neoplasia. 2000 Jul;5(3):295-306. doi: 10.1023/a:1009550912337. J Mammary Gland Biol Neoplasia. 2000. PMID: 14973392 Review.
-
Aryl hydrocarbon receptor: An emerging player in breast cancer pathogenesis and its potential as a drug target (Review).Mol Med Rep. 2024 Jan;29(1):11. doi: 10.3892/mmr.2023.13134. Epub 2023 Nov 24. Mol Med Rep. 2024. PMID: 37997818 Free PMC article. Review.
Cited by
-
A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERα protein.Carcinogenesis. 2012 May;33(5):1089-97. doi: 10.1093/carcin/bgs110. Epub 2012 Feb 16. Carcinogenesis. 2012. PMID: 22345291 Free PMC article.
-
Aminoflavone upregulates putative tumor suppressor miR-125b-2-3p to inhibit luminal A breast cancer stem cell-like properties.Precis Clin Med. 2022 Mar 28;5(2):pbac008. doi: 10.1093/pcmedi/pbac008. eCollection 2022 Jun. Precis Clin Med. 2022. PMID: 35694715 Free PMC article.
-
Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist.PLoS One. 2010 Nov 3;5(11):e13831. doi: 10.1371/journal.pone.0013831. PLoS One. 2010. PMID: 21072210 Free PMC article.
-
The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells.Cell Death Dis. 2014 Jan 30;5(1):e1038. doi: 10.1038/cddis.2013.549. Cell Death Dis. 2014. PMID: 24481452 Free PMC article.
-
Targeting mast cells tryptase in tumor microenvironment: a potential antiangiogenetic strategy.Biomed Res Int. 2014;2014:154702. doi: 10.1155/2014/154702. Epub 2014 Sep 11. Biomed Res Int. 2014. PMID: 25295247 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ 2008 Cancer statistics, 2008. CA Cancer J Clin 58:71–96 - PubMed
-
- U.S. Cancer Statistics Working Group 2009 United States cancer statistics: 1999–2005 Incidence and mortality web-based report. Atlanta, GA. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. (http://apps.nccd.cdc.gov/uscs)
-
- Bernard-Marty C, Cardoso F, Piccart MJ 2004 Facts and controversies in systemic treatment of metastic breast cancer. Oncologist 9:617–632 - PubMed
-
- Poland A, Knutson JC 1982 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 22:517–554 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

