Regulation of signal transducer and activator of transcription 3 enhanceosome formation by apurinic/apyrimidinic endonuclease 1 in hepatic acute phase response
- PMID: 20032196
- PMCID: PMC2817606
- DOI: 10.1210/me.2009-0319
Regulation of signal transducer and activator of transcription 3 enhanceosome formation by apurinic/apyrimidinic endonuclease 1 in hepatic acute phase response
Abstract
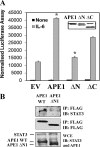
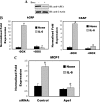
The signal transducer and activator of transcription-3 (STAT3) is a latent IL-6 inducible transcription factor that mediates hepatic and vascular inflammation. In this study, we make the novel observation that STAT3 forms an inducible complex with the apurinic/apyrimidinic endonuclease 1 (APE1)/redox effector factor-1 (APE1/Ref-1), an essential multifunctional protein in DNA base excision repair, and studied the role of APE1/Ref-1 in STAT3 function. Using a transfection-coimmunoprecipitation assay, we observed that APE1 selectively binds the NH(2)-terminal acetylation domain of STAT3. Ectopic expression of APE1 potentiated inducible STAT3 reporter activity, whereas knockdown of APE1 resulted in reduced IL-6-inducible acute-phase reactant protein expression (C-reactive protein and serum amyloid P) and monocyte chemotactic protein-1 expression. The mechanism for APE1 requirement in IL-6 signaling was indicated by reduced STAT3 DNA binding activity observed in response to small interfering RNA-mediated APE1 silencing. Consistent with these in vitro studies, we also observed that lipopolysaccharide-induced activation of acute-phase reactant protein expression is significantly abrogated in APE1 heterozygous mice compared with wild-type mice. IL-6 induces both STAT3 and APE1 to bind the suppressor of cytokine signaling-3 and gamma-fibrionogen promoters in their native chromatin environment. Moreover, we observed that APE1 knockdown destabilized formation of the STAT3-inducible enhanceosome on the endogenous gamma-fibrionogen promoter. Taken together, our study indicates that IL-6 induces a novel STAT3-APE1 complex, whose interaction is required for stable chromatin association in the IL-6-induced hepatic acute phase response.
Figures




Similar articles
-
The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression.J Biol Chem. 2007 Dec 21;282(51):37091-102. doi: 10.1074/jbc.M706458200. Epub 2007 Oct 23. J Biol Chem. 2007. PMID: 17956865
-
Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1.Oncogene. 2011 Jan 27;30(4):482-93. doi: 10.1038/onc.2010.435. Epub 2010 Sep 20. Oncogene. 2011. PMID: 20856196 Free PMC article.
-
APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3 dual-targeting effectively inhibits pancreatic cancer cell survival.PLoS One. 2012;7(10):e47462. doi: 10.1371/journal.pone.0047462. Epub 2012 Oct 19. PLoS One. 2012. PMID: 23094050 Free PMC article.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
-
APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions.Exp Mol Med. 2014 Jul 18;46(7):e106. doi: 10.1038/emm.2014.42. Exp Mol Med. 2014. PMID: 25033834 Free PMC article. Review.
Cited by
-
Radiation-induced C-reactive protein triggers apoptosis of vascular smooth muscle cells through ROS interfering with the STAT3/Ref-1 complex.J Cell Mol Med. 2022 Apr;26(7):2104-2118. doi: 10.1111/jcmm.17233. Epub 2022 Feb 17. J Cell Mol Med. 2022. PMID: 35178859 Free PMC article.
-
IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms.Mol Immunol. 2022 Jun;146:50-56. doi: 10.1016/j.molimm.2022.04.003. Epub 2022 Apr 14. Mol Immunol. 2022. PMID: 35430542 Free PMC article.
-
Therapeutic Lowering of C-Reactive Protein.Front Immunol. 2021 Jan 29;11:619564. doi: 10.3389/fimmu.2020.619564. eCollection 2020. Front Immunol. 2021. PMID: 33633738 Free PMC article. Review.
-
Suppression of STAT3 NH2 -terminal domain chemosensitizes medulloblastoma cells by activation of protein inhibitor of activated STAT3 via de-repression by microRNA-21.Mol Carcinog. 2018 Apr;57(4):536-548. doi: 10.1002/mc.22778. Epub 2018 Jan 25. Mol Carcinog. 2018. PMID: 29280516 Free PMC article.
-
Defining the functional potential and active community members of a sediment microbial community in a high-arctic hypersaline subzero spring.Appl Environ Microbiol. 2013 Jun;79(12):3637-48. doi: 10.1128/AEM.00153-13. Epub 2013 Apr 5. Appl Environ Microbiol. 2013. PMID: 23563939 Free PMC article.
References
-
- Levy DE, Darnell Jr JE 2002 Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3:651–662 - PubMed
-
- Darnell Jr JE, Kerr IM, Stark GR 1994 Jak-STAT pathways and transcritptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421 - PubMed
-
- Darnell Jr JE 1997 STATs and gene regulation. Science 277:1630–1635 - PubMed
-
- Schindler C, Darnell Jr JE 1995 Transcriptional response to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–651 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

