Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever
- PMID: 20032219
- PMCID: PMC2815520
- DOI: 10.1128/CVI.00454-09
Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever
Abstract
The world's largest Q fever outbreak is ongoing in The Netherlands with around 3,000 confirmed cases since the first half of 2007. Increased awareness has resulted in early referral of patients for diagnostics. An important drawback to serological diagnosis of acute Q fever is the lag phase in antibody response. Therefore, we evaluated the performance of a real-time PCR for detection of Coxiella burnetii DNA using serum samples from patients with acute Q fever. PCR, targeting IS1111, was retrospectively performed on acute-phase and follow-up convalescent-phase serum samples from 65 patients with acute Q fever as diagnosed by immunofluorescence assay. The results obtained by PCR were related to disease stage as defined by subsequent appearance of phase II IgM, phase II IgG, phase I IgM, and phase I IgG (IgM-II, IgG-II, IgM-I, and IgG-I, respectively) antibodies and time since onset of disease. In addition, we analyzed seronegative acute-phase serum samples from patients with inconclusive Q fever serology, because no convalescent-phase serum samples were available. PCR was scored positive in 49/50 (98%) seronegative sera, 9/10 (90%) sera with isolated IgM-II antibodies, 3/13 (23%) sera with IgM-II/IgG-II antibodies, 2/41 (5%) sera with IgM-II/IgG-II/IgM-I antibodies, 0/15 (0%) sera with IgM-II/IgG-II/IgM-I/IgG-I antibodies, and 0/1 (0%) serum sample with IgM-II/IgG-II/IgG-I antibodies. The latest time point after onset of disease in which C. burnetii DNA could be detected was at day 17. In patients with inconclusive Q fever serology, PCR was positive in 5/50 (10%) cases. We conclude that real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. C. burnetii DNA becomes undetectable in serum as the serological response develops.
Figures
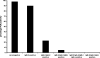

References
-
- de Wit, N. C. J., C. P. C. de Jager, J. C. E. Meekelenkamp, M. Schoorl, A. B. Gageldonk-Lafeber, A. C. A. P. Leenders, et al. 2009. Markers of infection in inpatients and outpatiens with acute Q-fever. Clin. Chem. Lab. Med. 47:1407-1409. - PubMed
-
- Karagiannis, I., G. Morroy, A. Rietveld, A. M. Horrevorts, M. Hamans, P. Francken, et al. 2007. Q fever outbreak in the Netherlands: a preliminary report. Euro Surveill. 12:E070809.2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

