Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity)
- PMID: 20032233
- PMCID: PMC2887637
- DOI: 10.1152/jn.00933.2009
Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity)
Abstract
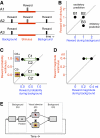
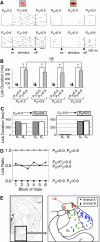
Prediction about outcomes constitutes a basic mechanism underlying informed economic decision making. A stimulus constitutes a reward predictor when it provides more information about the reward than the environmental background. Reward prediction can be manipulated in two ways, by varying the reward paired with the stimulus, as done traditionally in neurophysiological studies, and by varying the background reward while holding stimulus-reward pairing constant. Neuronal mechanisms involved in reward prediction should also be sensitive to changes in background reward independently of stimulus-reward pairing. We tested this assumption on a major brain structure involved in reward processing, the central and basolateral amygdala. In a 2 x 2 design, we examined the influence of rewarded and unrewarded backgrounds on neuronal responses to rewarded and unrewarded visual stimuli. Indeed, responses to the unchanged rewarded stimulus depended crucially on background reward in a population of amygdala neurons. Elevating background reward to the level of the rewarded stimulus extinguished these responses, and lowering background reward again reinstated the responses without changes in stimulus-reward pairing. None of these neurons responded specifically to an inhibitory stimulus predicting less reward compared with background (negative contingency). A smaller group of amygdala neurons maintained stimulus responses irrespective of background reward, possibly reflecting stimulus-reward pairing or visual sensory processes without reward prediction. Thus in being sensitive to background reward, the responses of a population of amygdala neurons to phasic stimuli appeared to follow the full criteria for excitatory reward prediction (positive contingency) rather than reflecting simple stimulus-reward pairing (contiguity).
Figures






References
-
- Aggleton JP, Passingham RE. Stereotaxic surgery under X-ray guidance in the rhesus monkey, with special reference to the amygdala. Exp Brain Res 44: 271–276, 1981 - PubMed
-
- Baker AG. Conditioned inhibition arising from a between-sessions negative correlation. J Exp Psychol 3: 144–155, 1977
-
- Balsam PD, Fairhurst S, Gallistel CR. Pavlovian contingencies and temporal information. J Exp Psychol Anim Behav Proc 32: 284–294, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

