The functional anatomy of a perceptual decision in the human brain
- PMID: 20032247
- PMCID: PMC2887630
- DOI: 10.1152/jn.00364.2009
The functional anatomy of a perceptual decision in the human brain
Abstract
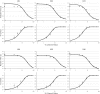
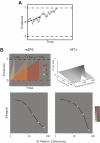
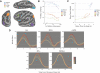
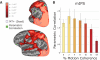
Our ability to make rapid decisions based on sensory information belies the complexity of the underlying computations. Recently, "accumulator" models of decision making have been shown to explain the activity of parietal neurons as macaques make judgments concerning visual motion. Unraveling the operation of a decision-making circuit, however, involves understanding both the responses of individual components in the neural circuitry and the relationships between them. In this functional magnetic resonance imaging study of the decision process in humans, we demonstrate that an accumulator model predicts responses to visual motion in the intraparietal sulcus (IPS). Significantly, the metrics used to define responses within the IPS also reveal distinct but interacting nodes in a circuit, including early sensory detectors in visual cortex, the visuomotor integration system of the IPS, and centers of cognitive control in the prefrontal cortex, all of which collectively define a perceptual decision-making network.
Figures



Similar articles
-
How embodied is perceptual decision making? Evidence for separate processing of perceptual and motor decisions.J Neurosci. 2013 Jan 30;33(5):2121-36. doi: 10.1523/JNEUROSCI.2334-12.2013. J Neurosci. 2013. PMID: 23365248 Free PMC article.
-
Neural representations of relevant and irrelevant features in perceptual decision making.J Neurosci. 2010 Nov 24;30(47):15778-89. doi: 10.1523/JNEUROSCI.3163-10.2010. J Neurosci. 2010. PMID: 21106817 Free PMC article.
-
Sharpened cortical tuning and enhanced cortico-cortical communication contribute to the long-term neural mechanisms of visual motion perceptual learning.Neuroimage. 2015 Jul 15;115:17-29. doi: 10.1016/j.neuroimage.2015.04.041. Epub 2015 Apr 25. Neuroimage. 2015. PMID: 25921327
-
Human cortical areas underlying the perception of optic flow: brain imaging studies.Int Rev Neurobiol. 2000;44:269-92. doi: 10.1016/s0074-7742(08)60746-1. Int Rev Neurobiol. 2000. PMID: 10605650 Review.
-
[Parietal Association Area and Motion Information Processing].Brain Nerve. 2016 Nov;68(11):1335-1343. doi: 10.11477/mf.1416200597. Brain Nerve. 2016. PMID: 27852024 Review. Japanese.
Cited by
-
A differentiation account of recognition memory: evidence from fMRI.J Cogn Neurosci. 2013 Mar;25(3):421-35. doi: 10.1162/jocn_a_00292. Epub 2012 Oct 23. J Cogn Neurosci. 2013. PMID: 23092213 Free PMC article.
-
Spatial encoding and underlying circuitry in scene-selective cortex.Neuroimage. 2013 Dec;83:892-900. doi: 10.1016/j.neuroimage.2013.07.030. Epub 2013 Jul 17. Neuroimage. 2013. PMID: 23872156 Free PMC article.
-
Memory accumulation mechanisms in human cortex are independent of motor intentions.J Neurosci. 2014 May 14;34(20):6993-7006. doi: 10.1523/JNEUROSCI.3911-13.2014. J Neurosci. 2014. PMID: 24828652 Free PMC article.
-
The contribution of the human posterior parietal cortex to episodic memory.Nat Rev Neurosci. 2017 Feb 17;18(3):183-192. doi: 10.1038/nrn.2017.6. Nat Rev Neurosci. 2017. PMID: 28209980 Free PMC article. Review.
-
High quality but limited quantity perceptual evidence produces neural accumulation in frontal and parietal cortex.Cereb Cortex. 2011 Nov;21(11):2650-62. doi: 10.1093/cercor/bhr055. Epub 2011 Apr 14. Cereb Cortex. 2011. PMID: 21498405 Free PMC article.
References
-
- Anderson JR. How Can the Human Mind Occur in the Physical Universe? New York: Oxford Univ. Press, 2007
-
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25: 27–31, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

