Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function
- PMID: 20032263
- PMCID: PMC2838659
- DOI: 10.1152/ajpregu.00367.2009
Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function
Erratum in
- Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1447
Abstract
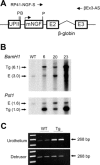
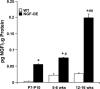
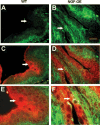
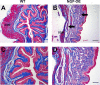
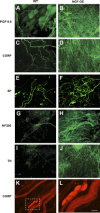
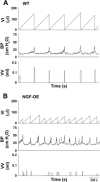
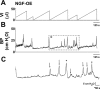
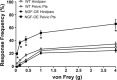
NGF has been suggested to play a role in urinary bladder dysfunction by mediating inflammation, as well as morphological and functional changes, in sensory and sympathetic neurons innervating the urinary bladder. To further explore the role of NGF in bladder sensory function, we generated a transgenic mouse model of chronic NGF overexpression in the bladder using the urothelium-specific uroplakin II (UPII) promoter. NGF mRNA and protein were expressed at higher levels in the bladders of NGF-overexpressing (NGF-OE) transgenic mice compared with wild-type littermate controls from postnatal day 7 through 12-16 wk of age. Overexpression of NGF led to urinary bladder enlargement characterized by marked nerve fiber hyperplasia in the submucosa and detrusor smooth muscle and elevated numbers of tissue mast cells. There was a marked increase in the density of CGRP- and substance P-positive C-fiber sensory afferents, neurofilament 200-positive myelinated sensory afferents, and tyrosine hydroxylase-positive sympathetic nerve fibers in the suburothelial nerve plexus. CGRP-positive ganglia were also present in the urinary bladders of transgenic mice. Transgenic mice had reduced urinary bladder capacity and an increase in the number and amplitude of nonvoiding bladder contractions under baseline conditions in conscious open-voiding cystometry. These changes in urinary bladder function were further associated with an increased referred somatic pelvic hypersensitivity. Thus, chronic urothelial NGF overexpression in transgenic mice leads to neuronal proliferation, focal increases in urinary bladder mast cells, increased urinary bladder reflex activity, and pelvic hypersensitivity. NGF-overexpressing mice may, therefore, provide a useful transgenic model for exploring the role of NGF in urinary bladder dysfunction.
Figures


Comment in
-
Model for chronic overexpression of NGF challenges old paradigms: focus on "overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function".Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R532-3. doi: 10.1152/ajpregu.00001.2010. Epub 2010 Jan 20. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20089712 No abstract available.
References
-
- Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49: 52–57, 1997 - PubMed
-
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci 110: 175–191, 2006 - PubMed
-
- Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J Neuroimmunol 18: 1–12, 1988 - PubMed
-
- Andreev NY, Dimitrieva N, Koltzenburg M, McMahon SB. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain 63: 109–115, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

