Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants
- PMID: 20032282
- PMCID: PMC2827520
- DOI: 10.2337/dc09-1770
Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants
Abstract
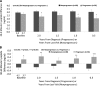
OBJECTIVE We studied the C-peptide response to oral glucose with progression to type 1 diabetes in Diabetes Prevention Trial-Type 1 (DPT-1) participants. RESEARCH DESIGN AND METHODS Among 504 DPT-1 participants <15 years of age, longitudinal analyses were performed in 36 progressors and 80 nonprogressors. Progressors had oral glucose tolerance tests (OGTTs) at baseline and every 6 months from 2.0 to 0.5 years before diagnosis; nonprogressors had OGTTs over similar intervals before their last visit. Sixty-six progressors and 192 nonprogressors were also studied proximal to and at diagnosis. RESULTS The 30-0 min C-peptide difference from OGTTs performed 2.0 years before diagnosis in progressors was lower than the 30-0 min C-peptide difference from OGTTs performed 2.0 years before the last visit in nonprogressors (P < 0.01) and remained lower over time. The 90-60 min C-peptide difference was positive at every OGTT before diagnosis in progressors, whereas it was negative at every OGTT before the last visit in nonprogressors (P < 0.01 at 2.0 years). The percentage whose peak C-peptide occurred at 120 min was higher in progressors at 2.0 years (P < 0.05); this persisted over time (P < 0.001 at 0.5 years). However, the peak C-peptide levels were only significantly lower at 0.5 years in progressors (P < 0.01). The timing of the peak C-peptide predicted type 1 diabetes (P < 0.001); peak C-peptide levels were less predictive (P < 0.05). CONCLUSIONS A decreased early C-peptide response to oral glucose and an increased later response occur at least 2 years before the diagnosis of type 1 diabetes.
Figures

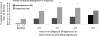

References
-
- Sherry NA, Tsai EB, Herold KC: Natural history of β-cell function in type 1 diabetes. Diabetes 2005;54 (Suppl. 2):S32–S39 - PubMed
-
- Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care 2006;29:643–649 - PubMed
-
- Skyler JS: Prediction and prevention of type 1 diabetes. Clin Pharm Ther 2007;81:768–771 - PubMed
-
- Eisenbarth GS: Update in type 1 diabetes. J Clin Endocrinol Metab 2007;92:2403–2407 - PubMed
-
- Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Diabetes Prevention Trial–Type 1 Study Group. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes. Diabetes Care 2007;30:38–42 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

