Interplay between ER exit code and domain conformation in CFTR misprocessing and rescue
- PMID: 20032308
- PMCID: PMC2820424
- DOI: 10.1091/mbc.e09-05-0427
Interplay between ER exit code and domain conformation in CFTR misprocessing and rescue
Abstract
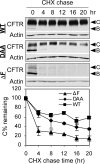
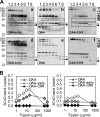
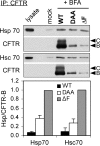
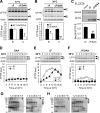
Multiple mutations in cystic fibrosis transmembrane conductance regulator (CFTR) impair its exit from the endoplasmic reticulum (ER). We compared two processing mutants: DeltaF508 and the ER exit code mutant DAA. Although both have severe kinetic processing defect, DAA but not DeltaF508 has substantial accumulation in its mature form, leading to higher level of processing at the steady state. DAA has much less profound conformational abnormalities. It has lower Hsp70 association and higher post-ER stability than DeltaF508. The ER exit code is necessary for DeltaF508 residual export and rescue. R555K, a mutation that rescues DeltaF508 misprocessing, improves Sec24 association and enhances its post-ER stability. Using in situ limited proteolysis, we demonstrated a clear change in trypsin sensitivity in DeltaF508 NBD1, which is reversed, together with that of other domains, by low temperature, R555K or both. We observed a conversion of the proteolytic pattern of DAA from the one resembling DeltaF508 to the one similar to wild-type CFTR during its maturation. Low temperature and R555K are additive in improving DeltaF508 conformational maturation and processing. Our data reveal a dual contribution of ER exit code and domain conformation to CFTR misprocessing and underscore the importance of conformational repair in effective rescue of DeltaF508.
Figures




Similar articles
-
Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments.J Biol Chem. 2001 Mar 23;276(12):8942-50. doi: 10.1074/jbc.M009172200. Epub 2000 Dec 21. J Biol Chem. 2001. PMID: 11124952
-
COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code.J Cell Biol. 2004 Oct 11;167(1):65-74. doi: 10.1083/jcb.200401035. J Cell Biol. 2004. PMID: 15479737 Free PMC article.
-
Diffusional mobility of the cystic fibrosis transmembrane conductance regulator mutant, delta F508-CFTR, in the endoplasmic reticulum measured by photobleaching of GFP-CFTR chimeras.J Biol Chem. 2002 May 10;277(19):16419-25. doi: 10.1074/jbc.M112361200. Epub 2002 Feb 27. J Biol Chem. 2002. PMID: 11877404
-
Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi.FEBS J. 2013 Sep;280(18):4396-406. doi: 10.1111/febs.12392. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23773658 Review.
-
Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum.Biochim Biophys Acta. 1999 Dec 6;1461(2):275-83. doi: 10.1016/s0005-2736(99)00163-7. Biochim Biophys Acta. 1999. PMID: 10581361 Review.
Cited by
-
Human heat shock protein 105/110 kDa (Hsp105/110) regulates biogenesis and quality control of misfolded cystic fibrosis transmembrane conductance regulator at multiple levels.J Biol Chem. 2012 Jun 1;287(23):19158-70. doi: 10.1074/jbc.M111.297580. Epub 2012 Apr 13. J Biol Chem. 2012. PMID: 22505710 Free PMC article.
-
Multiple evolutionarily conserved Di-leucine like motifs in the carboxyl terminus control the anterograde trafficking of NKCC2.J Biol Chem. 2012 Dec 14;287(51):42642-53. doi: 10.1074/jbc.M112.399162. Epub 2012 Oct 26. J Biol Chem. 2012. PMID: 23105100 Free PMC article.
-
Targets for cystic fibrosis therapy: proteomic analysis and correction of mutant cystic fibrosis transmembrane conductance regulator.Expert Rev Proteomics. 2010 Aug;7(4):495-506. doi: 10.1586/epr.10.45. Expert Rev Proteomics. 2010. PMID: 20653506 Free PMC article. Review.
-
Human-mouse cystic fibrosis transmembrane conductance regulator (CFTR) chimeras identify regions that partially rescue CFTR-ΔF508 processing and alter its gating defect.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):917-22. doi: 10.1073/pnas.1120065109. Epub 2011 Dec 30. Proc Natl Acad Sci U S A. 2012. PMID: 22210114 Free PMC article.
-
How to Avoid a No-Deal ER Exit.Cells. 2019 Sep 7;8(9):1051. doi: 10.3390/cells8091051. Cells. 2019. PMID: 31500301 Free PMC article. Review.
References
-
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M., Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Carlile G. W., Robert R., Zhang D., Teske K. A., Luo Y., Hanrahan J. W., Thomas D. Y. Correctors of protein trafficking defects identified by a novel high-throughput screening assay. Chembiochemistry. 2007;8:1012–1020. - PubMed
-
- Chang X. B., Cui L., Hou Y. X., Jensen T. J., Aleksandrov A. A., Mengos A., Riordan J. R. Removal of multiple arginine-framed trafficking signals overcomes misprocessing of delta F508 CFTR present in most patients with cystic fibrosis. Mol. Cell. 1999;4:137–142. - PubMed
-
- Chen E. Y., Bartlett M. C., Loo T. W., Clarke D. M. The DeltaF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2004;279:39620–39627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

