Beta arcades: recurring motifs in naturally occurring and disease-related amyloid fibrils
- PMID: 20032312
- PMCID: PMC2879952
- DOI: 10.1096/fj.09-145979
Beta arcades: recurring motifs in naturally occurring and disease-related amyloid fibrils
Abstract
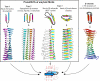
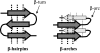
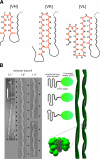
Amyloid fibrils are filamentous protein aggregates that accumulate in diseases such as Alzheimer's or type II diabetes. The amyloid-forming protein is disease specific. Amyloids may also be formed in vitro from many other proteins, after first denaturing them. Unlike the diverse native folds of these proteins, their amyloids are fundamentally similar in being rigid, smooth-sided, and cross-beta-structured, that is, with beta strands running perpendicular to the fibril axis. In the absence of high-resolution fibril structures, increasingly credible models are being derived by integrating data from a crossfire of experimental techniques. Most current models of disease-related amyloids invoke "beta arcades," columnar structures produced by in-register stacking of "beta arches." A beta arch is a strand-turn-strand motif in which the two beta strands interact via their side chains, not via the polypeptide backbone as in a conventional beta hairpin. Crystal structures of beta-solenoids, a class of proteins with amyloid-like properties, offer insight into the beta-arc turns found in beta arches. General conformational and thermodynamic considerations suggest that complexes of 2 or more beta arches may nucleate amyloid fibrillogenesis in vivo. The apparent prevalence of beta arches and their components have implications for identifying amyloidogenic sequences, elucidating fibril polymorphisms, predicting the locations and conformations of beta arcs within amyloid fibrils, and refining existing fibril models.
Figures

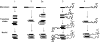

Similar articles
-
Standard conformations of beta-arches in beta-solenoid proteins.J Mol Biol. 2006 May 12;358(4):1094-105. doi: 10.1016/j.jmb.2006.02.039. Epub 2006 Mar 2. J Mol Biol. 2006. PMID: 16580019
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
-
Site-specific identification of non-beta-strand conformations in Alzheimer's beta-amyloid fibrils by solid-state NMR.Biophys J. 2003 May;84(5):3326-35. doi: 10.1016/S0006-3495(03)70057-5. Biophys J. 2003. PMID: 12719262 Free PMC article.
-
Synchrotron X-ray studies suggest that the core of the transthyretin amyloid fibril is a continuous beta-sheet helix.Structure. 1996 Aug 15;4(8):989-98. doi: 10.1016/s0969-2126(96)00104-9. Structure. 1996. PMID: 8805583
-
A brief overview of amyloids and Alzheimer's disease.Protein Sci. 2014 Oct;23(10):1315-31. doi: 10.1002/pro.2524. Epub 2014 Jul 30. Protein Sci. 2014. PMID: 25042050 Free PMC article. Review.
Cited by
-
Designed hairpin peptides interfere with amyloidogenesis pathways: fibril formation and cytotoxicity inhibition, interception of the preamyloid state.Biochemistry. 2011 Sep 27;50(38):8202-12. doi: 10.1021/bi200760h. Epub 2011 Aug 30. Biochemistry. 2011. PMID: 21848289 Free PMC article.
-
Cryo-EM fibril structures from systemic AA amyloidosis reveal the species complementarity of pathological amyloids.Nat Commun. 2019 Mar 7;10(1):1104. doi: 10.1038/s41467-019-09033-z. Nat Commun. 2019. PMID: 30846696 Free PMC article.
-
Usage of a dataset of NMR resolved protein structures to test aggregation versus solubility prediction algorithms.Protein Sci. 2017 Sep;26(9):1864-1869. doi: 10.1002/pro.3225. Epub 2017 Jul 15. Protein Sci. 2017. PMID: 28685932 Free PMC article.
-
Peptide from NSP7 is able to form amyloid-like fibrils: Artifact or challenge to drug design?Biochim Biophys Acta Proteins Proteom. 2023 Feb 1;1871(2):140884. doi: 10.1016/j.bbapap.2022.140884. Epub 2022 Dec 1. Biochim Biophys Acta Proteins Proteom. 2023. PMID: 36462605 Free PMC article.
-
Protein nanofibrils and their use as building blocks of sustainable materials.RSC Adv. 2021 Dec 8;11(62):39188-39215. doi: 10.1039/d1ra06878d. eCollection 2021 Dec 6. RSC Adv. 2021. PMID: 35492452 Free PMC article. Review.
References
-
- Pepys M B. Amyloidosis. Annu Rev Med. 2006;57:223–241. - PubMed
-
- Stefani M, Dobson C M. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81:678–699. - PubMed
-
- Lashuel H A, Lansbury P T., Jr Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys. 2006;39:167–201. - PubMed
-
- King C Y, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

