Control of steroid 21-oic acid synthesis by peroxisome proliferator-activated receptor alpha and role of the hypothalamic-pituitary-adrenal axis
- PMID: 20032461
- PMCID: PMC2844213
- DOI: 10.1074/jbc.M109.090175
Control of steroid 21-oic acid synthesis by peroxisome proliferator-activated receptor alpha and role of the hypothalamic-pituitary-adrenal axis
Abstract
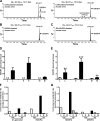
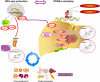
A previous study identified the peroxisome proliferator-activated receptor alpha (PPARalpha) activation biomarkers 21-steroid carboxylic acids 11beta-hydroxy-3,20-dioxopregn-4-en-21-oic acid (HDOPA) and 11beta,20-dihydroxy-3-oxo-pregn-4-en-21-oic acid (DHOPA). In the present study, the molecular mechanism and the metabolic pathway of their production were determined. The PPARalpha-specific time-dependent increases in HDOPA and 20alpha-DHOPA paralleled the development of adrenal cortex hyperplasia, hypercortisolism, and spleen atrophy, which was attenuated in adrenalectomized mice. Wy-14,643 activation of PPARalpha induced hepatic FGF21, which caused increased neuropeptide Y and agouti-related protein mRNAs in the hypothalamus, stimulation of the agouti-related protein/neuropeptide Y neurons, and activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in increased adrenal cortex hyperplasia and corticosterone production, revealing a link between PPARalpha and the HPA axis in controlling energy homeostasis and immune regulation. Corticosterone was demonstrated as the precursor of 21-carboxylic acids both in vivo and in vitro. Under PPARalpha activation, the classic reductive metabolic pathway of corticosterone was suppressed, whereas an alternative oxidative pathway was uncovered that leads to the sequential oxidation on carbon 21 resulting in HDOPA. The latter was then reduced to the end product 20alpha-DHOPA. Hepatic cytochromes P450, aldehyde dehydrogenase (ALDH3A2), and 21-hydroxysteroid dehydrogenase (AKR1C18) were found to be involved in this pathway. Activation of PPARalpha resulted in the induction of Aldh3a2 and Akr1c18, both of which were confirmed as target genes through introduction of promoter luciferase reporter constructs into mouse livers in vivo. This study underscores the power of mass spectrometry-based metabolomics combined with genomic and physiologic analyses in identifying downstream metabolic biomarkers and the corresponding upstream molecular mechanisms.
Figures







Similar articles
-
Metabolomics reveals an essential role for peroxisome proliferator-activated receptor α in bile acid homeostasis.J Lipid Res. 2012 Aug;53(8):1625-35. doi: 10.1194/jlr.M027433. Epub 2012 Jun 4. J Lipid Res. 2012. PMID: 22665165 Free PMC article.
-
Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation.Mol Endocrinol. 2007 Sep;21(9):2136-51. doi: 10.1210/me.2007-0150. Epub 2007 Jun 5. Mol Endocrinol. 2007. PMID: 17550978 Free PMC article.
-
Time course investigation of PPARalpha- and Kupffer cell-dependent effects of WY-14,643 in mouse liver using microarray gene expression.Toxicol Appl Pharmacol. 2007 Dec 15;225(3):267-77. doi: 10.1016/j.taap.2007.08.028. Epub 2007 Sep 16. Toxicol Appl Pharmacol. 2007. PMID: 17950772 Free PMC article.
-
PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators.Toxicology. 2008 Apr 3;246(1):2-8. doi: 10.1016/j.tox.2007.09.030. Epub 2007 Oct 7. Toxicology. 2008. PMID: 18006136 Review.
-
Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by PPARα.Int J Mol Sci. 2021 Aug 20;22(16):8969. doi: 10.3390/ijms22168969. Int J Mol Sci. 2021. PMID: 34445672 Free PMC article. Review.
Cited by
-
Peroxisome proliferator-activated receptor alpha target genes.PPAR Res. 2010;2010:612089. doi: 10.1155/2010/612089. Epub 2010 Sep 26. PPAR Res. 2010. PMID: 20936127 Free PMC article.
-
Nuclear receptor control of enterohepatic circulation.Compr Physiol. 2012 Oct;2(4):2811-28. doi: 10.1002/cphy.c120007. Compr Physiol. 2012. PMID: 23720266 Free PMC article. Review.
-
Transcriptional Profiles of Mitochondrial Dynamics Markers Are Disturbed in Adrenal Glands of Stressed Adult Male Rats.Life (Basel). 2023 Jun 27;13(7):1457. doi: 10.3390/life13071457. Life (Basel). 2023. PMID: 37511832 Free PMC article.
-
Metabolomics: an essential tool to understand the function of peroxisome proliferator-activated receptor alpha.Toxicol Pathol. 2013 Feb;41(2):410-8. doi: 10.1177/0192623312466960. Epub 2012 Nov 28. Toxicol Pathol. 2013. PMID: 23197196 Free PMC article. Review.
-
Metabolomics reveals an essential role for peroxisome proliferator-activated receptor α in bile acid homeostasis.J Lipid Res. 2012 Aug;53(8):1625-35. doi: 10.1194/jlr.M027433. Epub 2012 Jun 4. J Lipid Res. 2012. PMID: 22665165 Free PMC article.
References
-
- Desvergne B., Wahli W. (1999) Endocr. Rev. 20, 649–688 - PubMed
-
- Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Cell Metab. 5, 415–425 - PubMed
-
- Nicholson J. K., Lindon J. C., Holmes E. (1999) Xenobiotica 29, 1181–1189 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

