Effect of lipid particle biogenesis on the subcellular distribution of squalene in the yeast Saccharomyces cerevisiae
- PMID: 20032462
- PMCID: PMC2825407
- DOI: 10.1074/jbc.M109.074229
Effect of lipid particle biogenesis on the subcellular distribution of squalene in the yeast Saccharomyces cerevisiae
Abstract
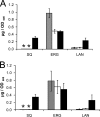
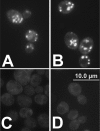
Squalene belongs to the group of isoprenoids and is a precursor for the synthesis of sterols, steroids, and ubiquinones. In the yeast Saccharomyces cerevisiae, the amount of squalene can be increased by variation of growth conditions or by genetic manipulation. In this report, we show that a hem1Delta mutant accumulated a large amount of squalene, which was stored almost exclusively in cytoplasmic lipid particles/droplets. Interestingly, a strain bearing a hem1Delta deletion in a dga1Delta lro1Delta are1Delta are2Delta quadruple mutant background (QMhem1Delta), which is devoid of the classical storage lipids, triacylglycerols and steryl esters, and lacks lipid particles, accumulated squalene at similar amounts as the hem1Delta mutant in a wild type background. In QMhem1Delta, however, increased amounts of squalene were found in cellular membranes, especially in microsomes. The fact that QMhem1Delta did not form lipid particles indicated that accumulation of squalene solely was not sufficient to initiate proliferation of lipid particles. Most importantly, these results also demonstrated that (i) squalene was not lipotoxic under the conditions tested, and (ii) organelle membranes in yeast can accommodate relatively large quantities of this non-polar lipid without compromising cellular functions. In summary, localization of squalene as described here can be regarded as an unconventional example of non-polar lipid storage in cellular membranes.
Figures


Similar articles
-
Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 2012 Apr;1821(4):647-53. doi: 10.1016/j.bbalip.2012.01.015. Epub 2012 Feb 8. Biochim Biophys Acta. 2012. PMID: 22342273 Free PMC article.
-
Squalene is lipotoxic to yeast cells defective in lipid droplet biogenesis.Biochem Biophys Res Commun. 2016 Jan 22;469(4):1123-8. doi: 10.1016/j.bbrc.2015.12.050. Epub 2015 Dec 15. Biochem Biophys Res Commun. 2016. PMID: 26703208
-
A yeast strain lacking lipid particles bears a defect in ergosterol formation.J Biol Chem. 2004 Jul 23;279(30):31190-6. doi: 10.1074/jbc.M403251200. Epub 2004 May 21. J Biol Chem. 2004. PMID: 15155725
-
Synthesis and turnover of non-polar lipids in yeast.Prog Lipid Res. 2008 May;47(3):157-71. doi: 10.1016/j.plipres.2008.01.001. Epub 2008 Jan 18. Prog Lipid Res. 2008. PMID: 18258205 Review.
-
Yeast lipid metabolism at a glance.FEMS Yeast Res. 2014 May;14(3):369-88. doi: 10.1111/1567-1364.12141. Epub 2014 Mar 5. FEMS Yeast Res. 2014. PMID: 24520995 Review.
Cited by
-
Plant sterol metabolism. Δ(7)-Sterol-C5-desaturase (STE1/DWARF7), Δ(5,7)-sterol-Δ(7)-reductase (DWARF5) and Δ(24)-sterol-Δ(24)-reductase (DIMINUTO/DWARF1) show multiple subcellular localizations in Arabidopsis thaliana (Heynh) L.PLoS One. 2013;8(2):e56429. doi: 10.1371/journal.pone.0056429. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409184 Free PMC article.
-
Lipid droplets and peroxisomes: key players in cellular lipid homeostasis or a matter of fat--store 'em up or burn 'em down.Genetics. 2013 Jan;193(1):1-50. doi: 10.1534/genetics.112.143362. Genetics. 2013. PMID: 23275493 Free PMC article. Review.
-
Lipid saturation induces degradation of squalene epoxidase for sterol homeostasis and cell survival.Life Sci Alliance. 2022 Nov 11;6(1):e202201612. doi: 10.26508/lsa.202201612. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36368908 Free PMC article.
-
Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 2012 Apr;1821(4):647-53. doi: 10.1016/j.bbalip.2012.01.015. Epub 2012 Feb 8. Biochim Biophys Acta. 2012. PMID: 22342273 Free PMC article.
-
The Plasma Membrane H+-ATPase Promoter Driving the Expression of FADX Enables Highly Efficient Production of Punicic Acid in Rhodotorula toruloides Cultivated on Glucose and Crude Glycerol.J Fungi (Basel). 2024 Sep 13;10(9):649. doi: 10.3390/jof10090649. J Fungi (Basel). 2024. PMID: 39330409 Free PMC article.
References
-
- Rissmann R., Oudshoorn M. H., Kocks E., Hennink W. E., Ponec M., Bouwstra J. A. (2008) Biochim. Biophys. Acta 1778, 2350–2360 - PubMed
-
- Kohno Y., Egawa Y., Itoh S., Nagaoka S., Takahashi M., Mukai K. (1995) Biochim. Biophys. Acta 1256, 52–56 - PubMed
-
- Ko T. F., Weng Y. M., Chiou R. Y. (2002) J. Agric. Food Chem. 50, 5343–5348 - PubMed
-
- Qureshi A. A., Lehmann J. W., Peterson D. M. (1996) J. Nutr. 126, 1972–1978 - PubMed
-
- Ivashkevich S. P., Apukhovskaia L. I., Vendt V. P. (1981) Biokhimiia 46, 1420–1425 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

