Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation
- PMID: 20032466
- PMCID: PMC2820802
- DOI: 10.1074/jbc.M109.026203
Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation
Abstract
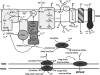
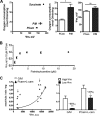
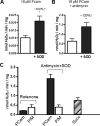
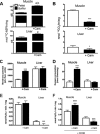
Oxidative stress in skeletal muscle is a hallmark of various pathophysiologic states that also feature increased reliance on long-chain fatty acid (LCFA) substrate, such as insulin resistance and exercise. However, little is known about the mechanistic basis of the LCFA-induced reactive oxygen species (ROS) burden in intact mitochondria, and elucidation of this mechanistic basis was the goal of this study. Specific aims were to determine the extent to which LCFA catabolism is associated with ROS production and to gain mechanistic insights into the associated ROS production. Because intermediates and by-products of LCFA catabolism may interfere with antioxidant mechanisms, we predicted that ROS formation during LCFA catabolism reflects a complex process involving multiple sites of ROS production as well as modified mitochondrial function. Thus, we utilized several complementary approaches to probe the underlying mechanism(s). Using skeletal muscle mitochondria, our findings indicate that even a low supply of LCFA is associated with ROS formation in excess of that generated by NADH-linked substrates. Moreover, ROS production was evident across the physiologic range of membrane potential and was relatively insensitive to membrane potential changes. Determinations of topology and membrane potential as well as use of inhibitors revealed complex III and the electron transfer flavoprotein (ETF) and ETF-oxidoreductase, as likely sites of ROS production. Finally, ROS production was sensitive to matrix levels of LCFA catabolic intermediates, indicating that mitochondrial export of LCFA catabolic intermediates can play a role in determining ROS levels.
Figures
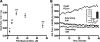
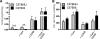
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

