Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study
- PMID: 20032498
- PMCID: PMC2930903
- DOI: 10.1182/blood-2009-06-230250
Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study
Abstract
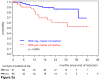
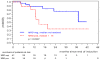
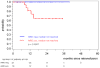
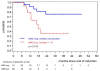
The prognostic impact of minimal residual disease (MRD) was analyzed in 259 patients with mantle cell lymphoma (MCL) treated within 2 randomized trials of the European MCL Network (MCL Younger and MCL Elderly trial). After rituximab-based induction treatment, 106 of 190 evaluable patients (56%) achieved a molecular remission (MR) based on blood and/or bone marrow (BM) analysis. MR resulted in a significantly improved response duration (RD; 87% vs 61% patients in remission at 2 years, P = .004) and emerged to be an independent prognostic factor for RD (hazard ratio = 0.4, 95% confidence interval, 0.1-0.9, P = .028). MR was highly predictive for prolonged RD independent of clinical response (complete response [CR], complete response unconfirmed [CRu], partial response [PR]; RD at 2 years: 94% in BM MRD-negative CR/CRu and 100% in BM MRD-negative PR, compared with 71% in BM MRD-positive CR/CRu and 51% in BM MRD-positive PR, P = .002). Sustained MR during the postinduction period was predictive for outcome in MCL Younger after autologous stem cell transplantation (ASCT; RD at 2 years 100% vs 65%, P = .001) and during maintenance in MCL Elderly (RD at 2 years: 76% vs 36%, P = .015). ASCT increased the proportion of patients in MR from 55% before high-dose therapy to 72% thereafter. Sequential MRD monitoring is a powerful predictor for treatment outcome in MCL. These trials are registered at www.clinicaltrials.gov as #NCT00209222 and #NCT00209209.
Keywords: Adult; Aged; Aged, 80 and over; Antineoplastic Combined Chemotherapy Protocols; Cell Separation; Combined Modality Therapy; Female; Flow Cytometry; Humans; Immunohistochemistry; Immunotherapy; Lymphoma, Mantle-Cell; MRD; Male; Middle Aged; Neoplasm Staging; Neoplasm, Residual; Polymerase Chain Reaction; Prognosis; RQ-PCR; Radiotherapy; Treatment Outcome; autologous stem cell transplantation; immunochemotherapy; mantle cell lymphoma; methods; minimal residual disease; pathology; therapeutic use; therapy.
Conflict of interest statement
Figures






Comment in
-
The adulthood of MRD detection in MCL.Blood. 2010 Apr 22;115(16):3180-1. doi: 10.1182/blood-2010-01-262733. Blood. 2010. PMID: 20413661 No abstract available.
Similar articles
-
Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma.Blood. 2006 Mar 15;107(6):2271-8. doi: 10.1182/blood-2005-07-2845. Epub 2005 Dec 6. Blood. 2006. PMID: 16332971
-
Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network.Lancet. 2016 Aug 6;388(10044):565-75. doi: 10.1016/S0140-6736(16)00739-X. Epub 2016 Jun 14. Lancet. 2016. PMID: 27313086 Clinical Trial.
-
Predictive Value of Minimal Residual Disease for Efficacy of Rituximab Maintenance in Mantle Cell Lymphoma: Results From the European Mantle Cell Lymphoma Elderly Trial.J Clin Oncol. 2024 Feb 10;42(5):538-549. doi: 10.1200/JCO.23.00899. Epub 2023 Nov 22. J Clin Oncol. 2024. PMID: 37992261
-
Immunotherapy with rituximab following high-dose therapy and autologous stem-cell transplantation for mantle cell lymphoma.Semin Oncol. 2002 Feb;29(1 Suppl 2):56-69. Semin Oncol. 2002. PMID: 11842390 Review.
-
Refining the Mantle Cell Lymphoma Paradigm: Impact of Novel Therapies on Current Practice.Clin Cancer Res. 2015 Sep 1;21(17):3853-61. doi: 10.1158/1078-0432.CCR-15-0488. Epub 2015 Jun 9. Clin Cancer Res. 2015. PMID: 26059189 Review.
Cited by
-
Ofatumumab plus HyperCVAD/HD-MA induction leads to high rates of minimal residual disease negativity in patients with newly diagnosed mantle cell lymphoma: Results of a phase 2 study.Cancer. 2022 Apr 15;128(8):1595-1604. doi: 10.1002/cncr.34106. Epub 2022 Feb 14. Cancer. 2022. PMID: 35157306 Free PMC article. Clinical Trial.
-
Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders.Leukemia. 2014 Jun;28(6):1299-307. doi: 10.1038/leu.2013.375. Epub 2013 Dec 17. Leukemia. 2014. PMID: 24342950
-
Hematopoietic cell transplantation for mantle cell lymphoma.Int J Hematol. 2022 Mar;115(3):301-309. doi: 10.1007/s12185-022-03294-z. Epub 2022 Jan 29. Int J Hematol. 2022. PMID: 35092557 Review.
-
Biological and clinical determinants shaping heterogeneity in mantle cell lymphoma.Blood Adv. 2024 Jul 23;8(14):3652-3664. doi: 10.1182/bloodadvances.2023011763. Blood Adv. 2024. PMID: 38748869 Free PMC article. Review.
-
VcR-CVAD Induction Chemotherapy Followed by Maintenance Rituximab Produces Durable Remissions in Mantle Cell Lymphoma: A Wisconsin Oncology Network Study.Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):e61-e67. doi: 10.1016/j.clml.2017.10.006. Epub 2017 Nov 4. Clin Lymphoma Myeloma Leuk. 2018. PMID: 29191715 Free PMC article.
References
-
- Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–518. - PubMed
-
- Meusers P, Engelhard M, Bartels H, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematol Oncol. 1989;7(5):365–380. - PubMed
-
- Siebert R, Matthiesen P, Harder S, et al. Application of interphase cytogenetics for the detection of t(11;14)(q13;q32) in mantle cell lymphomas. Ann Oncol. 1998;9(5):519–526. - PubMed
-
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the german low grade lymphoma study group (GLSG) J Clin Oncol. 2005;23(9):1984–1992. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

