CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress
- PMID: 20032512
- PMCID: PMC2838582
- DOI: 10.1152/ajpcell.00153.2009
CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress
Abstract
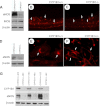
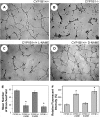
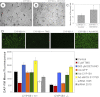
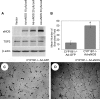
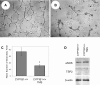
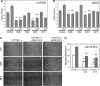
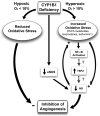
We have recently shown that deletion of constitutively expressed CYP1B1 is associated with attenuation of retinal endothelial cell (EC) capillary morphogenesis (CM) in vitro and angiogenesis in vivo. This was largely caused by increased intracellular oxidative stress and increased production of thrombospondin-2, an endogenous inhibitor of angiogenesis. Here, we demonstrate that endothelium nitric oxide synthase (eNOS) expression is dramatically decreased in the ECs prepared from retina, lung, heart, and aorta of CYP1B1-deficient (CYP1B1(-/-)) mice compared with wild-type (CYP1B1(+/+)) mice. The eNOS expression was also decreased in retinal vasculature of CYP1B1(-/-) mice. Inhibition of eNOS activity in cultured CYP1B1(+/+) retinal ECs blocked CM and was concomitant with increased oxidative stress, like in CYP1B1(-/-) retinal ECs. In addition, expression of eNOS in CYP1B1(-/-) retinal ECs or their incubation with a nitric oxide (NO) donor enhanced NO levels, lowered oxidative stress, and improved cell migration and CM. Inhibition of CYP1B1 activity in the CYP1B1(+/+) retinal ECs resulted in reduced NO levels and attenuation of CM. In contrast, expression of CYP1B1 increased NO levels and enhanced CM of CYP1B1(-/-) retinal ECs. Furthermore, attenuation of CYP1B1 expression with small interfering RNA proportionally lowered eNOS expression and NO levels in wild-type cells. Together, our results link CYP1B1 metabolism in retinal ECs with sustained eNOS activity and NO synthesis and/or bioavailability and low oxidative stress and thrombospondin-2 expression. Thus CYP1B1 and eNOS cooperate in different ways to lower oxidative stress and thereby to promote CM in vitro and angiogenesis in vivo.
Figures


References
-
- Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, Saikia J, Kim M, Melia M, Fishman M, Huang P, Campochiaro PA. Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol 191: 116–124, 2002 - PubMed
-
- Beauchamp MH, Sennlaub F, Speranza G, Gobeil JF, Checchin D, Kermorvant-Duchemin E, Abran D, Hardy P, Lachapelle P. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med 37: 1885–1894, 2004 - PubMed
-
- Bejjani BA, Stockton DW, Lewis RA, Tomey KF, Dueker DK, Jabak M, Astle WF, Lupski JR. Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus. Hum Mol Genet 9: 367–374, 2000 - PubMed
-
- Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem 270: 11595–11602, 1995 - PubMed
-
- Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72: 683–689, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

