Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane
- PMID: 20032513
- PMCID: PMC2838566
- DOI: 10.1152/ajpcell.00455.2009
Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane
Abstract
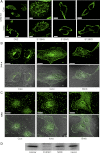
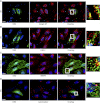
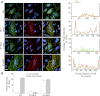
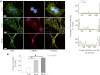
Tumor protein D52 (also known as CRHSP-28) is highly expressed in multiple cancers and tumor-derived cell lines; however, it is normally abundant in secretory epithelia throughout the digestive system, where it has been implicated in Ca(2+)-dependent digestive enzyme secretion (41). Here we demonstrate, using site-specific mutations, that Ca(2+)-sensitive phosphorylation at serine 136 modulates the accumulation of D52 at the plasma membrane within 2 min of cell stimulation. When expressed in Chinese hamster ovary CHO-K1 cells, D52 colocalized with adaptor protein AP-3, Rab27A, vesicle-associated membrane protein VAMP7, and lysosomal-associated membrane protein LAMP1, all of which are present in lysosome-like secretory organelles. Overexpression of D52 resulted in a marked accumulation of LAMP1 on the plasma membrane that was further enhanced following elevation of cellular Ca(2+). Strikingly, mutation of serine 136 to alanine abolished the Ca(2+)-stimulated accumulation of LAMP1 at the plasma membrane whereas phosphomimetic mutants constitutively induced LAMP1 plasma membrane accumulation independent of elevated Ca(2+). Identical results were obtained for endogenous D52 in normal rat kidney and HeLA cells, where both LAMP1 and D52 rapidly accumulated on the plasma membrane in response to elevated cellular Ca(2+). Finally, D52 induced the uptake of LAMP1 antibodies from the cell surface in accordance with both the level of D52 expression and phosphorylation at serine 136 demonstrating that D52 altered the plasma membrane recycling of LAMP1-associated secretory vesicles. These findings implicate both D52 expression and Ca(2+)-dependent phosphorylation at serine 136 in lysosomal membrane trafficking to and from the plasma membrane providing a novel Ca(2+)-sensitive pathway modulating the lysosome-like secretory pathway.
Figures




Similar articles
-
Tumor protein D52 controls trafficking of an apical endolysosomal secretory pathway in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2013 Sep 15;305(6):G439-52. doi: 10.1152/ajpgi.00143.2013. Epub 2013 Jul 18. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23868405 Free PMC article.
-
A role for tumor protein TPD52 phosphorylation in endo-membrane trafficking during cytokinesis.Biochem Biophys Res Commun. 2010 Nov 26;402(4):583-7. doi: 10.1016/j.bbrc.2010.10.041. Epub 2010 Oct 12. Biochem Biophys Res Commun. 2010. PMID: 20946871 Free PMC article.
-
Calcium/calmodulin-dependent phosphorylation of tumor protein D52 on serine residue 136 may be mediated by CAMK2delta6.Am J Physiol Gastrointest Liver Physiol. 2008 Dec;295(6):G1159-72. doi: 10.1152/ajpgi.90345.2008. Epub 2008 Oct 2. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18832449 Free PMC article.
-
Detection of Lysosomal Exocytosis by Surface Exposure of Lamp1 Luminal Epitopes.Methods Mol Biol. 2017;1594:205-211. doi: 10.1007/978-1-4939-6934-0_13. Methods Mol Biol. 2017. PMID: 28456985
-
Different localization of lysosomal-associated membrane protein 1 (LAMP1) in mammalian cultured cell lines.Histochem Cell Biol. 2020 Apr;153(4):199-213. doi: 10.1007/s00418-019-01842-z. Epub 2020 Jan 6. Histochem Cell Biol. 2020. PMID: 31907597
Cited by
-
Tumor protein D52 controls trafficking of an apical endolysosomal secretory pathway in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2013 Sep 15;305(6):G439-52. doi: 10.1152/ajpgi.00143.2013. Epub 2013 Jul 18. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23868405 Free PMC article.
-
TPD52 expression increases neutral lipid storage within cultured cells.J Cell Sci. 2015 Sep 1;128(17):3223-38. doi: 10.1242/jcs.167692. Epub 2015 Jul 16. J Cell Sci. 2015. PMID: 26183179 Free PMC article.
-
Secretion of novel SEL1L endogenous variants is promoted by ER stress/UPR via endosomes and shed vesicles in human cancer cells.PLoS One. 2011 Feb 17;6(2):e17206. doi: 10.1371/journal.pone.0017206. PLoS One. 2011. PMID: 21359144 Free PMC article.
-
Genetics, Cell Biology, and Pathophysiology of Pancreatitis.Gastroenterology. 2019 May;156(7):1951-1968.e1. doi: 10.1053/j.gastro.2018.11.081. Epub 2019 Jan 18. Gastroenterology. 2019. PMID: 30660731 Free PMC article. Review.
-
Tumor protein D52 (TPD52) and cancer-oncogene understudy or understudied oncogene?Tumour Biol. 2014 Aug;35(8):7369-82. doi: 10.1007/s13277-014-2006-x. Epub 2014 May 6. Tumour Biol. 2014. PMID: 24798974 Review.
References
-
- Ashrafi K, Chang FY, Watts JL, Faser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272, 2003 - PubMed
-
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, DasGupta R, Perrimon N, Malhotra V. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439: 604–607, 2006 - PubMed
-
- Boutros R, Bailey AM, Wilson SHD, Byrne JA. Alternative splicing as a mechanism for regulating 14-3-3 binding: interactions between hD53 (TPD52L1) and 14-3-3 proteins. J Mol Biol 332: 675–687, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

