Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety
- PMID: 20032541
- PMCID: PMC2879645
- DOI: 10.3851/IMP1463
Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety
Abstract
Background: A crucial gap in the development of microbicides for HIV prevention is the absence of models predictive of safety. Previous studies have demonstrated an increased susceptibility to genital herpes in mice following repeated applications of nonoxynol-9 (N-9). This study was designed to explore the underlying mechanisms, focusing on the effects that N-9 has on genital tract epithelium and to apply this expanded model to evaluate the safety of microbicides that have been advanced to clinical trials.
Methods: Mice were treated intravaginally with formulated 3.5% N-9, 1% tenofovir, 0.5% or 2% PRO 2000, hydroxyethylcellulose (HEC) placebo or no treatment and the effect on herpes simplex virus 2 (HSV-2) susceptibility, epithelial cell architecture, junctional proteins and inflammation were assessed.
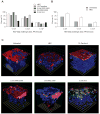
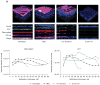
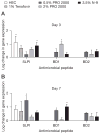
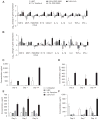
Results: Mice treated with seven daily doses of N-9, but not tenofovir, PRO 2000 or HEC, were significantly more susceptible to challenge with low doses of HSV-2; confocal microscopy demonstrated increased numbers of viral particles deep within the genital tract. N-9 disrupted the epithelium with loss of tight and adherens junctional proteins. By contrast, the epithelium was relatively preserved following tenofovir, PRO 2000 and HEC exposure. Additionally, N-9, but not the other microbicides, triggered a significant inflammatory response relative to untreated mice.
Conclusions: These findings indicate that disruption of the epithelium contributes to increased HSV-2 susceptibility and might provide a biomarker predictive of increased risk for HIV acquisition. The results are consistent with the safety outcomes of the recently completed Phase IIb clinical trial with 0.5% PRO 2000 gel, and predict that tenofovir gel will not adversely affect the genital tract.
Conflict of interest statement
The authors declare no competing interests.
Figures

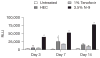
References
-
- Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. AIDS. 2000;14:85–88. - PubMed
-
- Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. - PubMed
-
- Cohen J. AIDS research. Promising AIDS vaccine’s failure leaves field reeling. Science. 2007;318:28–29. - PubMed
-
- HIV vaccine failure prompts Merck to halt trial. Nature. 2007;449:390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

