Synthesis, structural studies and antitumoral evaluation of C-6 alkyl and alkenyl side chain pyrimidine derivatives
- PMID: 20032865
- PMCID: PMC6255265
- DOI: 10.3390/molecules14124866
Synthesis, structural studies and antitumoral evaluation of C-6 alkyl and alkenyl side chain pyrimidine derivatives
Abstract
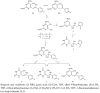
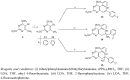
The synthetic route for introduction of fluorophenylalkyl (compounds 5, 7, 14 and 15) and fluorophenylalkenyl (compounds 4E and 13) side chains at C-6 of the pyrimidine nucleus involved the lithiation of the pyrimidine derivatives 1, 2 and 11 and subsequent nucleophilic addition or substitution reactions of the organolithium intermediate thus obtained with 2-fluorophenylacetone, 4-fluoroacetophenone or ethyl 4-fluorobenzoate as electrophiles. The structures of novel compounds were confirmed by (1)H-, (19)F- and (13)C-NMR and MS. Compounds 8 and 10 containing unsaturated fluorophenylalkyl side chains showed better inhibitory effect than their saturated fluorophenylalkylated pyrimidine counterparts 7 and 9. A conformational study based on NOE enhancements showed the importance of the double bond and substitution in the side chain for the conformational preferences in relation to inhibitory activity. Among all tested compounds, C-5 furyl (12) and phenyl (13 and 15) substituted pyrimidine derivatives showed significant cytostatic activities against all tested tumor cell lines.
Figures



References
-
- Dolbier W.R., Jr. Fluorine chemistry at the millennium. J. Fluorine Chem. 2005;126:157–163. doi: 10.1016/j.jfluchem.2004.09.033. - DOI
-
- Isanbor C., O'Hagan D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor. Chem. 2006;127:303–319. doi: 10.1016/j.jfluchem.2006.01.011. - DOI
-
- Howard J.A.K., Hoy V.J., O'Hagan D., Smith G.T. How good is fluorine as a hydrogen bond acceptor? Tetrahedron. 1996;52:12613–12622. doi: 10.1016/0040-4020(96)00749-1. - DOI
-
- Bergstrom D.E., Swartling D.J. Structure, Reactivity, Synthesis and Applications. In: Liebman J.F., Greenberg A., Dolbier W.R. Jr., editors. Fluorine-Containing Molecules. VCH; New York, NY, USA: 1988. pp. 259–308.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

