Microwave multicomponent synthesis
- PMID: 20032870
- PMCID: PMC6254856
- DOI: 10.3390/molecules14124936
Microwave multicomponent synthesis
Abstract
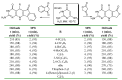
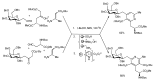
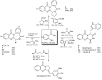
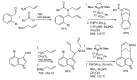
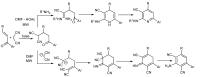
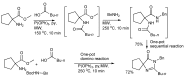
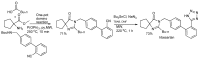
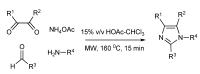
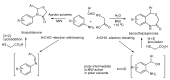
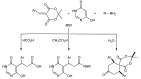
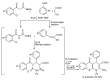
In the manner that very important research is often performed by multidisciplinary research teams, the applications of multicomponent reactions involving the combination of multiple starting materials with different functional groups leading to the higher efficiency and environmentally friendly construction of multifunctional/complex target molecules is growing in importance. This review will explore the advances and advantages in microwave multicomponent synthesis (MMS) that have been achieved over the last five years.
Figures
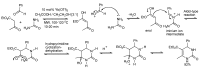
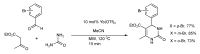

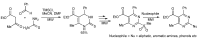
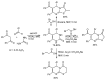
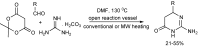
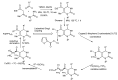
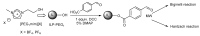
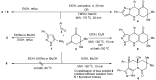
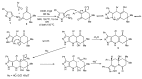



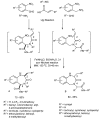
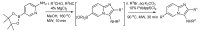
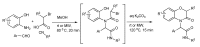
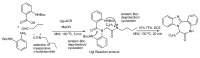

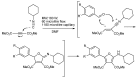
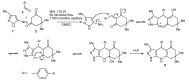
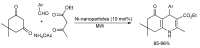

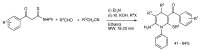

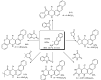

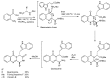
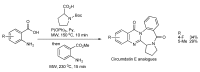
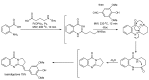
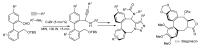

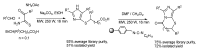
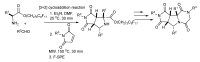
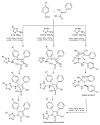
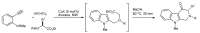
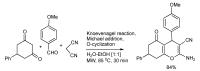
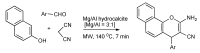
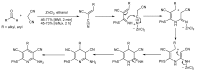
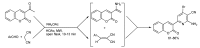
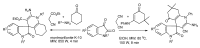
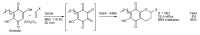
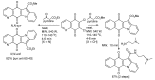
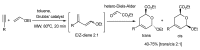
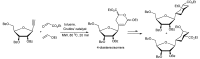
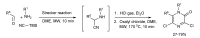
References
-
- Loupy A. Microwaves in Organic Synthesis. Vol. 2. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2006. pp. 788–819.
-
- Bagley M.C., Lubinu M.C. Microwave-assisted multicomponent reactions for the synthesis of heterocycles. Top Heterocycl. Chem. 2006;1:31–58. doi: 10.1007/7081_004. - DOI
-
- Sujatha K., Shanmugam P., Perumal P.T., Muralidharan D., Rajendran M. Synthesis and cardiac effects of 3,4-dihydropyrimidin-2-(1H)-one-5 Carboxylates. Bioorg. Med. Chem. Lett. 2006;16:4893–4897. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

