Neuroprotection by radical avoidance: search for suitable agents
- PMID: 20032877
- PMCID: PMC6255388
- DOI: 10.3390/molecules14125054
Neuroprotection by radical avoidance: search for suitable agents
Abstract
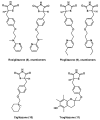
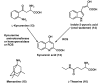
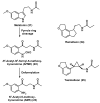
Neurodegeneration is frequently associated with damage by free radicals. However, increases in reactive oxygen and nitrogen species, which may ultimately lead to neuronal cell death, do not necessarily reflect its primary cause, but can be a consequence of otherwise induced cellular dysfunction. Detrimental processes which promote free radical formation are initiated, e.g., by disturbances in calcium homeostasis, mitochondrial malfunction, and an age-related decline in the circadian oscillator system. Free radicals generated at high rates under pathophysiological conditions are insufficiently detoxified by scavengers. Interventions at the primary causes of dysfunction, which avoid secondary rises in radical formation, may be more efficient. The aim of such approaches should be to prevent calcium overload, to reduce mitochondrial electron dissipation, to support electron transport capacity, and to avoid circadian perturbations. L-theanine and several amphiphilic nitrones are capable of counteracting excitotoxicity and/or mitochondrial radical formation. Resveratrol seems to promote mitochondrial biogenesis. Mitochondrial effects of leptin include attenuation of electron leakage. Melatonin combines all the requirements mentioned, additionally regulates anti- and pro-oxidant enzymes and is, with few exceptions, very well tolerated. In this review, the perspectives, problems and limits of drugs are compared which may be suitable for reducing the formation of free radicals.
Figures


Similar articles
-
Melatonin defeats neurally-derived free radicals and reduces the associated neuromorphological and neurobehavioral damage.J Physiol Pharmacol. 2007 Dec;58 Suppl 6:5-22. J Physiol Pharmacol. 2007. PMID: 18212398 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity.Ann N Y Acad Sci. 2003 May;993:197-207; discussion 287-8. doi: 10.1111/j.1749-6632.2003.tb07530.x. Ann N Y Acad Sci. 2003. PMID: 12853314 Review.
-
Melatonin: a well-documented antioxidant with conditional pro-oxidant actions.J Pineal Res. 2014 Sep;57(2):131-46. doi: 10.1111/jpi.12162. Epub 2014 Aug 6. J Pineal Res. 2014. PMID: 25060102 Review.
-
Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes.Neurotox Res. 2013 Apr;23(3):267-300. doi: 10.1007/s12640-012-9337-4. Epub 2012 Jun 28. Neurotox Res. 2013. PMID: 22739839 Review.
Cited by
-
Melatonin and Nitrones As Potential Therapeutic Agents for Stroke.Front Aging Neurosci. 2016 Nov 23;8:281. doi: 10.3389/fnagi.2016.00281. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27932976 Free PMC article.
-
Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways.Biomolecules. 2021 Jul 9;11(7):1002. doi: 10.3390/biom11071002. Biomolecules. 2021. PMID: 34356626 Free PMC article. Review.
-
Neurodegeneration, memory loss, and dementia: the impact of biological clocks and circadian rhythm.Front Biosci (Landmark Ed). 2021 Sep 30;26(9):614-627. doi: 10.52586/4971. Front Biosci (Landmark Ed). 2021. PMID: 34590471 Free PMC article. Review.
-
Toxic Synergism Between Quinolinic Acid and Glutaric Acid in Neuronal Cells Is Mediated by Oxidative Stress: Insights to a New Toxic Model.Mol Neurobiol. 2018 Jun;55(6):5362-5376. doi: 10.1007/s12035-017-0761-6. Epub 2017 Sep 21. Mol Neurobiol. 2018. PMID: 28936789
-
Melatonin and the electron transport chain.Cell Mol Life Sci. 2017 Nov;74(21):3883-3896. doi: 10.1007/s00018-017-2615-9. Epub 2017 Aug 7. Cell Mol Life Sci. 2017. PMID: 28785805 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

