Synthesis of chiral 1,4-disubstituted-1,2,3-triazole derivatives from amino acids
- PMID: 20032880
- PMCID: PMC6255254
- DOI: 10.3390/molecules14125124
Synthesis of chiral 1,4-disubstituted-1,2,3-triazole derivatives from amino acids
Abstract
A versatile method for the synthesis of chiral 1,4-disubstituted-1,2,3-triazole derivatives starting from easily accessible naturally occurring D-or L-amino acids as chiral synthons is described. The amino acids were converted into azido alcohols, followed by copper catalyzed [3+2] cycloaddition reactions between the azido alcohols and methyl propiolate and subsequent ester aminolysis with primary and secondary amines furnished the target compounds, which were obtained in excellent yields with no racemization. Docking of selected target compounds shows that the chiral 1,4-disubstituted-1,2,3-triazoles derivatives has the potential of mimicking the binding mode of known purine analogues.
Figures

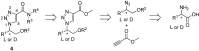

References
-
- Fan W.Q., Katritzky A.R. 1,2,3 Triazoles. In: Katritzky A.R., Rees C.W., Scriven E.F.V., editors. Comprehensive Heterocycle Chemistry II. Vol. 4. Pergamon Press; New York, NY, USA: 1996. pp. 1–126.
-
- Katritzky A.R., Zhang Y., Singh S.K. 1,2,3-Triazole formation under mild conditions via 1,3-dipolar cycloaddition of acetylenes with azides. Heterocycles. 2003;60:1225–1239. doi: 10.3987/REV-02-562. - DOI
-
- Alvarez R., Velazquez S., San R., Aquaro S., De C.E., Perno C.F., Karlsson A., Balzarini J., Camarasa M.J. 1,2,3-Triazole-[2',5'-bis-O-(tert-butyldimethylsilyl)-beta-D- ribofuranosyl]-3'-spiro-5"-(4"-amino-1",2"-oxathiole 2",2"-dioxide) (TSAO) analogues: Synthesis and anti-HIV-1 activity. J. Med. Chem. 1994;37:4185–4194. doi: 10.1021/jm00050a015. - DOI - PubMed
-
- Velazquez S., Alvarez R., Perez C., Gago F., De C.E., Balzarini J., Camarasa J.M. Regiospecific synthesis and anti-human immunodeficiency virus activity of novel 5-substituted N-alkylcarbamoyl and N,N-dialkylcarbamoyl 1,2,3-triazole-TSAO analogues. Antiviral Chem. Chemother. 1998;9:481–489. - PubMed
-
- Whiting M., Muldoon J., Lin Y.C., Silverman S.M., Lindstrom W., Olson A.J., Kolb H.C., Finn M.G., Sharpless B.K., Elder J.H., Fokin V.V. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006;45:1435–1439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

