Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression
- PMID: 20032992
- PMCID: PMC3057125
- DOI: 10.1038/jid.2009.384
Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression
Abstract
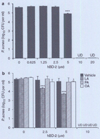
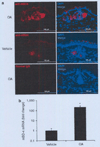
Various sebum free fatty acids (FFAs) have shown antibacterial activity against a broad range of gram-positive bacteria, resulting in the suggestion that they are accountable, at least partially, for the direct antimicrobial activity of the skin surface. In this study, we examined the effects of sebum FFAs on the antimicrobial peptide (AMP)-mediated innate immune defense of human sebocytes. Incubation of lauric acid, palmitic acid, or oleic acid (OA) with human sebocytes dramatically enhanced their expression of human beta-defensin (hBD)-2, one of the predominant AMPs found in the skin, whereas remarkable increases in hBD-1, hBD-3, and human cathelicidin LL-37 were not observed. Secreted hBD-2 was detectable by western blotting in the supernatant of sebocyte culture incubated with each FFA, but not with a vehicle control. The supernatant of FFA-incubated sebocyte culture showed antimicrobial activity against Propionibacterium acnes, whereas the enhanced antimicrobial activity of human sebocytes was neutralized by anti-hBD-2 IgG. In addition, the FFA-induced hBD-2 expression was suppressed by blocking the cluster of differentiation (CD)36 fatty acid translocase on the surface of sebocytes with anti-human CD36 IgG or blocking the NF-kappaB signaling pathway with BMS-345541, a highly selective inhibitor of inhibitory kappaB kinase. These data suggest that sebum FFAs upregulate the expression of hBD-2 in human sebocytes, which may enhance the disinfecting activity of the human sebaceous gland. The FFA-induced upregulation of hBD-2 is facilitated by CD36-mediated FFA uptake and NF-kappaB-mediated transactivation. The upregulation of mouse beta-defensin 4, a mouse ortholog for hBD-2, was also observed in the hair follicle sebaceous glands of mouse ear skin after an epicutaneous application of OA, the most hBD-2-inducible FFA tested. This report highlights the potential of using FFAs as a multifunctional antimicrobial therapy agent for acne vulgaris treatment; FFAs may provide direct antibacterial activities against P. acnes and enhance the skin's innate antibacterial defense by inducing the expression of hBD-2 in sebocytes as well.
Conflict of interest statement
The authors state no conflict of interest.
Figures




References
-
- Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. - PubMed
-
- Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. - PubMed
-
- Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. - PubMed
-
- Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase HPLC: chromatographic mobility as a function of physico-chemical properties. Int J Mol Sci. 2007;8:1111–1124.
-
- Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

