Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes
- PMID: 20033053
- PMCID: PMC2838235
- DOI: 10.1038/leu.2009.271
Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes
Abstract
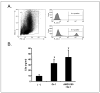
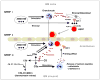
We reported that complement cascade (CC) becomes activated in bone marrow (BM) during mobilization of hematopoietic stem/progenitor cells (HSPCs) induced by granulocyte colony-stimulating factor (G-CSF) and C5 cleavage has an important function in optimal egress of HSPCs. In this work, we explored whether CC is involved in mobilization of HSPCs induced by the CXCR4 antagonist, AMD3100. To address this question, we performed mobilization studies in mice that display a defect in the activation of the proximal steps of CC (Rag(-/-), severe combined immune deficient (SCID), C2.Cfb(-/-)) as well as in mice that do not activate the distal steps of CC (C5(-/-)). We noticed that proximal CC activation-deficient mice (above C5 level), in contrast to distal step CC activation-deficient C5(-/-) ones, mobilize normally in response to AMD3100 administration. We hypothesized that this discrepancy in mobilization could be explained by AMD3100-activating C5 in Rag(-/-), SCID, and C2.Cfb(-/-) animals in a non-canonical mechanism involving activated granulocytes. To support this, granulocytes (i) first egress from BM and (ii) secrete several proteases that cleave/activate C5 in response to AMD3100. We conclude that AMD3100-directed mobilization of HSPCs, similarly to G-CSF-induced mobilization, depends on activation of CC; however, in contrast to G-CSF, AMD3100 activates the distal steps of CC directly at the C5 level. Overall, these data support that C5 cleavage fragments and distal steps of CC activation are required for optimal mobilization of HSPCs.
Figures


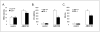
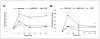
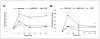

References
-
- Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139:94–100. - PubMed
-
- Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz) 2009;57:269–278. - PubMed
-
- Andrews RG, Briddell RA, Knitter GH, Opie T, Bronsden M, Myerson D, et al. In vivo synergy between recombinant human stem cell factor and recombinant human granulocyte colony-stimulating factor in baboons enhanced circulation of progenitor cells. Blood. 1994;84:800–810. - PubMed
-
- Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85:2269–2275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

