The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase!
- PMID: 20033063
- PMCID: PMC2824469
- DOI: 10.1038/emboj.2009.376
The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase!
Abstract
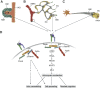
Dynamic interactions of cells with their environment regulate multiple aspects of tissue morphogenesis and function. Integrins are the major class of cell surface receptors that recognize and bind extracellular matrix proteins, resulting in the engagement and organization of the cytoskeleton as well as activation of signalling pathways to regulate cell behaviour and morphogenetic processes. The ternary complex of integrin-linked kinase (ILK), PINCH, and parvin (IPP complex), which was identified more than a decade ago, interacts with the cytoplasmic tail of beta integrins and couples them to the actin cytoskeleton. In addition, ILK has been shown to act as a serine/threonine kinase and to directly activate several signalling pathways downstream of integrins. However, the kinase activity of ILK and the precise functions of the IPP complex have remained elusive and controversial. This review focuses on the recent advances made towards understanding the specialized roles this complex and its individual components have acquired during evolution.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Abraham S, Kogata N, Fässler R, Adams RH (2008) Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res 102: 562–570 - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

