Gamma-vinyl GABA increases nonvesicular release of GABA and glutamate in the nucleus accumbens in rats via action on anion channels and GABA transporters
- PMID: 20033132
- PMCID: PMC3713230
- DOI: 10.1007/s00213-009-1753-7
Gamma-vinyl GABA increases nonvesicular release of GABA and glutamate in the nucleus accumbens in rats via action on anion channels and GABA transporters
Abstract
Rationale: gamma-Amino butyric acid (GABA) is a well-characterized inhibitory neurotransmitter in the central nervous system, which may also stimulate nonvesicular release of other neurotransmitters under certain conditions. We have recently reported that gamma-vinyl GABA (GVG), an irreversible GABA transaminase inhibitor, elevates extracellular GABA but fails to alter dopamine release in the nucleus accumbens (NAc).
Objectives: Here, we investigated the mechanism(s) by which GVG elevates extracellular GABA levels and whether GVG also alters glutamate release in the NAc.
Materials and methods: In vivo microdialysis was used to simultaneously measure extracellular NAc GABA and glutamate before and after GVG administration in freely moving rats.
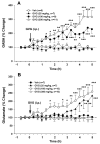
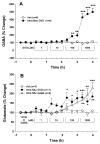
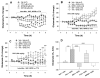
Results: Systemic administration of GVG or intra-NAc local perfusion of GVG significantly increased extracellular NAc GABA and glutamate. GVG-enhanced GABA was completely blocked by intra-NAc local perfusion of 5-nitro-2, 3-(phenylpropylamino)-benzoic acid (NPPB), a selective anion channel blocker and partially blocked by SKF89976A, a type 1 GABA transporter inhibitor. GVG-enhanced glutamate was completely blocked by NPPB or SKF89976A. Tetrodotoxin, a voltage-dependent Na(+)-channel blocker, failed to alter GVG-enhanced GABA and glutamate.
Conclusions: These data suggest that GVG-enhanced extracellular GABA and glutamate are mediated predominantly by the opening of anion channels and partially by the reversal of GABA transporters. Enhanced extracellular glutamate may functionally attenuate the pharmacological action of GABA and prevent enhanced GABA-induced excess inhibition.
Figures

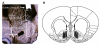
Similar articles
-
The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats.Neuropharmacology. 2008 Mar;54(3):542-51. doi: 10.1016/j.neuropharm.2007.11.005. Epub 2007 Nov 19. Neuropharmacology. 2008. PMID: 18155073 Free PMC article.
-
Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine.Synapse. 1999 Oct;34(1):11-9. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. Synapse. 1999. PMID: 10459167
-
Gamma-vinyl GABA inhibits cocaine-triggered reinstatement of drug-seeking behavior in rats by a non-dopaminergic mechanism.Drug Alcohol Depend. 2008 Oct 1;97(3):216-25. doi: 10.1016/j.drugalcdep.2007.10.004. Epub 2007 Dec 11. Drug Alcohol Depend. 2008. PMID: 18063319 Free PMC article.
-
Brain microdialysis of GABA and glutamate: what does it signify?Synapse. 1997 Nov;27(3):242-61. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. Synapse. 1997. PMID: 9329159 Review.
-
Microdialysis and the neurochemistry of addiction.Pharmacol Biochem Behav. 2008 Aug;90(2):261-72. doi: 10.1016/j.pbb.2007.09.001. Epub 2007 Sep 12. Pharmacol Biochem Behav. 2008. PMID: 17928041 Free PMC article. Review.
Cited by
-
Neuronal gamma-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA.Front Cell Neurosci. 2015 May 18;9:188. doi: 10.3389/fncel.2015.00188. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26041994 Free PMC article.
-
Cocaine-Induced Time-Dependent Alterations in Cytochrome P450 and Liver Function.Int J Mol Sci. 2023 Jan 13;24(2):1632. doi: 10.3390/ijms24021632. Int J Mol Sci. 2023. PMID: 36675146 Free PMC article. Review.
References
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
-
- Bonanno G, Raiteri M. Release-regulating presynaptic hetero-carriers. Prog Neurobiol. 1994;44:451–462. - PubMed
-
- Bonanno G, Pittaluga A, Fedele E, Fontana G, Raiteri M. Glutamic acid and aminobutyric acid modulate each other's release through heterocarriers sited on the axon terminals of rat brain. J Neurochem. 1993;61:222–230. - PubMed
-
- Bonanno G, Raiteri L, Paluzzi S, Zappettini S, Usai C, Raiteri M. Co-existence of GABA and Glu transporters in the central nervous system. Curr Top Med Chem. 2006;6:979–988. - PubMed
-
- Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

