Peripheral facial nerve axotomy in mice causes sprouting of motor axons into perineuronal central white matter: time course and molecular characterization
- PMID: 20034058
- PMCID: PMC4491910
- DOI: 10.1002/cne.22240
Peripheral facial nerve axotomy in mice causes sprouting of motor axons into perineuronal central white matter: time course and molecular characterization
Erratum in
- J Comp Neurol. 2012 Jun 1;520(8):ii. Pararajasingham, Abirami [corrected to Pararajasingam, Abirami]
Abstract
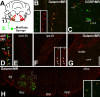
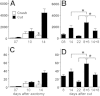
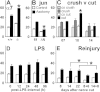
Generation of new axonal sprouts plays an important role in neural repair. In the current study, we examined the appearance, composition and effects of gene deletions on intrabrainstem sprouts following peripheral facial nerve axotomy. Axotomy was followed by the appearance of galanin(+) and calcitonin gene-related peptide (CGRP)(+) sprouts peaking at day 14, matching both large, neuropeptide(+) subpopulations of axotomized facial motoneurons, but with CGRP(+) sprouts considerably rarer. Strong immunoreactivity for vesicular acetylcholine transporter (VAChT) and retrogradely transported MiniRuby following its application on freshly cut proximal facial nerve stump confirmed their axotomized motoneuron origin; the sprouts expressed CD44 and alpha7beta1 integrin adhesion molecules and grew apparently unhindered along neighboring central white matter tracts. Quantification of the galanin(+) sprouts revealed a stronger response following cut compared with crush (day 7-14) as well as enhanced sprouting after recut (day 8 + 6 vs. 14; 14 + 8 vs. 22), arguing against delayed appearance of sprouting being the result of the initial phase of reinnervation. Sprouting was strongly diminished in brain Jun-deficient mice but enhanced in alpha7 null animals that showed apparently compensatory up-regulation in beta1, suggesting important regulatory roles for transcription factors and the sprout-associated adhesion molecules. Analysis of inflammatory stimuli revealed a 50% reduction 12-48 hours following systemic endotoxin associated with neural inflammation and a tendency toward more sprouts in TNFR1/2 null mutants (P = 10%) with a reduced inflammatory response, indicating detrimental effects of excessive inflammation. Moreover, the study points to the usefulness of the facial axotomy model in exploring physiological and molecular stimuli regulating central sprouting.
2009 Wiley-Liss, Inc.
Figures





References
-
- Bohatschek M, Werner A, Raivich G. Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol. 2001;172:137–152. - PubMed
-
- Bohatschek M, Kloss CU, Hristova M, Pfeffer K, Raivich G. Microglial major histocompatibility complex glycoprotein-1 in the axotomized facial motor nucleus: regulation and role of tumor necrosis factor receptors 1 and 2. J Comp Neurol. 2004;470:382–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

