Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy
- PMID: 20034063
- PMCID: PMC3098130
- DOI: 10.1002/cne.22235
Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy
Abstract
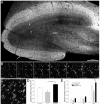
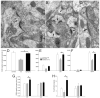
Many patients with temporal lobe epilepsy display neuron loss in the dentate gyrus. One potential epileptogenic mechanism is loss of GABAergic interneurons and inhibitory synapses with granule cells. Stereological techniques were used to estimate numbers of gephyrin-positive punctae in the dentate gyrus, which were reduced short-term (5 days after pilocarpine-induced status epilepticus) but later rebounded beyond controls in epileptic rats. Stereological techniques were used to estimate numbers of synapses in electron micrographs of serial sections processed for postembedding GABA-immunoreactivity. Adjacent sections were used to estimate numbers of granule cells and glutamic acid decarboxylase-positive neurons per dentate gyrus. GABAergic neurons were reduced to 70% of control levels short-term, where they remained in epileptic rats. Integrating synapse and cell counts yielded average numbers of GABAergic synapses per granule cell, which decreased short-term and rebounded in epileptic animals beyond control levels. Axo-shaft and axo-spinous GABAergic synapse numbers in the outer molecular layer changed most. These findings suggest interneuron loss initially reduces numbers of GABAergic synapses with granule cells, but later, synaptogenesis by surviving interneurons overshoots control levels. In contrast, the average number of excitatory synapses per granule cell decreased short-term but recovered only toward control levels, although in epileptic rats excitatory synapses in the inner molecular layer were larger than in controls. These findings reveal a relative excess of GABAergic synapses and suggest that reports of reduced functional inhibitory synaptic input to granule cells in epilepsy might be attributable not to fewer but instead to abundant but dysfunctional GABAergic synapses.
2009 Wiley-Liss, Inc.
Figures




References
-
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. - PubMed
-
- Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. - PubMed
-
- Arellano JI, Muñoz A, Ballesteros-Yáñnez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127:45–64. - PubMed
-
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol. 2004;476:205–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

