Sperm and oocyte communication mechanisms controlling C. elegans fertility
- PMID: 20034089
- PMCID: PMC2963114
- DOI: 10.1002/dvdy.22202
Sperm and oocyte communication mechanisms controlling C. elegans fertility
Abstract
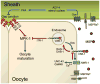
During sexual reproduction in many species, sperm and oocyte secrete diffusible signaling molecules to help orchestrate the biological symphony of fertilization. In the Caenorhabditis elegans gonad, bidirectional signaling between sperm and oocyte is important for guiding sperm to the fertilization site and inducing oocyte maturation. The molecular mechanisms that regulate sperm guidance and oocyte maturation are being delineated. Unexpectedly, these mechanisms are providing insight into human diseases, such as amyotrophic lateral sclerosis, spinal muscular atrophy, and cancer. Here we review sperm and oocyte communication in C. elegans and discuss relationships to human disorders.
Copyright (c) 2009 Wiley-Liss, Inc.
Figures



Similar articles
-
An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans.Genes Dev. 2003 Jan 15;17(2):187-200. doi: 10.1101/gad.1028303. Genes Dev. 2003. PMID: 12533508 Free PMC article.
-
Oocyte production and sperm utilization patterns in semi-fertile strains of Caenorhabditis elegans.BMC Dev Biol. 2004 Apr 15;4:3. doi: 10.1186/1471-213X-4-3. BMC Dev Biol. 2004. PMID: 15086962 Free PMC article.
-
Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans.Curr Biol. 2008 May 20;18(10):705-714. doi: 10.1016/j.cub.2008.04.043. Epub 2008 May 8. Curr Biol. 2008. PMID: 18472420 Free PMC article.
-
Every sperm is sacred: fertilization in Caenorhabditis elegans.Dev Biol. 2001 Feb 15;230(2):101-9. doi: 10.1006/dbio.2000.0118. Dev Biol. 2001. PMID: 11161565 Review.
-
Developmental control of oocyte maturation and egg activation in metazoan models.Cold Spring Harb Perspect Biol. 2011 Oct 1;3(10):a005553. doi: 10.1101/cshperspect.a005553. Cold Spring Harb Perspect Biol. 2011. PMID: 21709181 Free PMC article. Review.
Cited by
-
Eph receptor signaling in C. elegans.WormBook. 2012 Nov 29:1-17. doi: 10.1895/wormbook.1.151.1. WormBook. 2012. PMID: 23197476 Free PMC article. Review.
-
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging.Sci Rep. 2016 Aug 18;6:32021. doi: 10.1038/srep32021. Sci Rep. 2016. PMID: 27535493 Free PMC article.
-
A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract.PLoS Genet. 2013;9(1):e1003271. doi: 10.1371/journal.pgen.1003271. Epub 2013 Jan 31. PLoS Genet. 2013. PMID: 23382703 Free PMC article.
-
Visualization of the biphasic calcium wave during fertilization in Caenorhabditis elegans using a genetically encoded calcium indicator.Biol Open. 2023 Sep 15;12(9):bio059832. doi: 10.1242/bio.059832. Epub 2023 Aug 21. Biol Open. 2023. PMID: 37602653 Free PMC article.
-
Intense sperm-mediated sexual conflict promotes reproductive isolation in Caenorhabditis nematodes.PLoS Biol. 2014 Jul 29;12(7):e1001915. doi: 10.1371/journal.pbio.1001915. eCollection 2014 Jul. PLoS Biol. 2014. PMID: 25072732 Free PMC article.
References
-
- Aitken RJ, Kelly RW. Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil. 1985;73:139–146. - PubMed
-
- Anagnostou G, Akbar MT, Paul P, Angelinetta C, Steiner TJ, de Belleroche J. Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol Aging 2008 - PubMed
-
- Arehart E, Stitham J, Asselbergs FW, Douville K, MacKenzie T, Fetalvero KM, Gleim S, Kasza Z, Rao Y, Martel L, Segel S, Robb J, Kaplan A, Simons M, Powell RJ, Moore JH, Rimm EB, Martin KA, Hwa J. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ Res. 2008;102:986–993. - PMC - PubMed
-
- Arimura A, Yasui K, Kishino J, Asanuma F, Hasegawa H, Kakudo S, Ohtani M, Arita H. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J Pharmacol Exp Ther. 2001;298:411–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

