Survival of Hoxa13 homozygous mutants reveals a novel role in digit patterning and appendicular skeletal development
- PMID: 20034107
- PMCID: PMC2981150
- DOI: 10.1002/dvdy.22183
Survival of Hoxa13 homozygous mutants reveals a novel role in digit patterning and appendicular skeletal development
Abstract
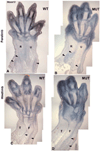
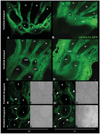
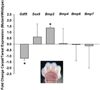
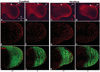
The loss of HOXA13 function severely disrupts embryonic limb development. However, because embryos lacking HOXA13 die by mid-gestation, the defects present in the mutant limb could arise as a secondary consequence of failing embryonic health. In our analysis of the mutant Hoxa13(GFP) allele, we identified a surviving cohort of homozygous mutants exhibiting severe limb defects including: missing phalanx elements, fusions of the carpal/tarsal elements, and significant reductions in metacarpal/metatarsal length. Characterization of prochondrogenic genes in the affected carpal/tarsal regions revealed significant reduction in Gdf5 expression, whereas Bmp2 expression was significantly elevated. Analysis of Gdf5 mRNA localization also revealed diffuse expression in the carpal/tarsal anlagen, suggesting a role for HOXA13 in the organization of the cells necessary to delineate individual carpal/tarsal elements. Together these results identify Gdf5 as a potential target gene of HOXA13 target gene and confirm a specific role for HOXA13 during appendicular skeletal development.
Figures



References
-
- Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. - PubMed
-
- Archer CW, Cottril C, Rooney P. Cellular aspects of cartilage differentiation and morphogenesis. In: Kemp R, Hinchliffe JR, editors. Matrices and differentiation. New York: A.R. Liss; 1984. p. 480. - PubMed
-
- Barna M, Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell. 2007;12:931–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

