Using ultrasonography to define fetal-maternal relationships: moving from humans to mice
- PMID: 20034427
- PMCID: PMC2799334
Using ultrasonography to define fetal-maternal relationships: moving from humans to mice
Abstract
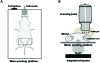
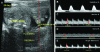
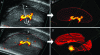
Ultrasound scanning is a noninvasive, accurate, and cost-effective method to create images of the female reproductive tract clinically and in research. Ultrasonography is particularly valuable for studying the dynamic relationships among mother, placenta, and fetus during pregnancy because this modality does not disturb the ongoing course of gestation. Importantly, the complex vascular changes in the mother induced by pregnancy and the vascular system generated to support placental function can be assessed quantitatively and functionally by ultrasonography. Many mouse models are available that address aspects of human placental function and dysfunction, but high-quality microultrasound technology suitable for use in pregnant mice has become widely available only recently. This technical advance now enables real-time recording of maternal-fetal interactions in pregnant rodents. The ability to perform microultrasonic analyses of parameters such as uterine arterial remodeling, hemodynamic changes, placental development, and fetal growth in mice now permits research that uses the same imaging platform as that for human patients. This capability will enhance the translation of information derived from rodent studies to the clinic.
Figures
References
-
- Abramowicz JS, Sheiner E. 2007. In utero imaging of the placenta: importance for diseases of pregnancy. Placenta 28:S14–S22 - PubMed
-
- Adamson SL. 2007. Placental vascular disease in mouse models of intrauterine growth restriction. Biol Reprod 77:69–b–69
-
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. 2002. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 250:358–373 - PubMed
-
- Akirav C, Lu Y, Mu J, Qu DW, Zhou YQ, Slevin J, Holmyard D, Foster FS, Adamson SL. 2005. Ultrasonic detection and developmental changes in calcification of the placenta during normal pregnancy in mice. Placenta 26:129–137 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
