Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted
- PMID: 20034470
- PMCID: PMC2817945
- DOI: 10.1016/j.bbrc.2009.12.090
Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted
Abstract
Mutations in the preproinsulin protein that affect processing of preproinsulin to proinsulin or lead to misfolding of proinsulin are associated with diabetes. We examined the subcellular localization and secretion of 13 neonatal diabetes-associated human proinsulin proteins (A24D, G32R, G32S, L35P, C43G, G47V, F48C, G84R, R89C, G90C, C96Y, S101C and Y108C) in rat INS-1 insulinoma cells. These mutant proinsulin proteins accumulate in the endoplasmic reticulum (ER) and are poorly secreted except for G84R and in contrast to wild-type and hyperproinsulinemia-associated mutant proteins (H34D and R89H) which were sorted to secretory granules and efficiently secreted. We also examined the effect of C96Y mutant proinsulin on the synthesis and secretion of wild-type insulin and observed a dominant-negative effect of the mutant proinsulin on the synthesis and secretion of wild-type insulin due to induction of the unfolded protein response and resulting attenuation of overall translation.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

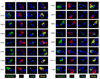

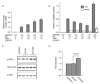
References
-
- Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI. Neonatal Diabetes International Collaborative Group, Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA. 2007;104:15040–15044. - PMC - PubMed
-
- Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI, Neonatal Diabetes International Collaborative Group. Hattersley AT, Ellard S. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034–1042. - PMC - PubMed
-
- Polak M, Dechaume A, Cavé H, Nimri R, Crosnier H, Sulmont V, de Kerdanet M, Scharfmann R, Lebenthal Y, Froguel P, Vaxillaire M. Heterozygous missense mutations in the insulin gene are linked to permanent diabetes appearing in the neonatal period or in early infancy. A report from the French ND (neonatal diabetes) study group. Diabetes. 2008;57:1115–1119. - PubMed
-
- Molven A, Ringdal M, Nordbø AM, Ræder H, Støy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, Undlien DE, Joner G, Søvik O, the Norwegian Childhood Diabetes Study Group. Bell GI, Njølstad PR. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131–1135. - PubMed
-
- Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, Hansen T, Federici L, Pesavento R, Cadario F, Federici G, Ghirri P, Arvan P, Iafusco D, Barbetti F and the Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (SIEDP) Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J. Clin. Invest. 2008;118:2148–2156. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

