Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes
- PMID: 20034607
- PMCID: PMC2866524
- DOI: 10.1016/j.vaccine.2009.12.015
Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes
Abstract
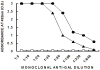
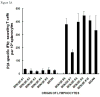
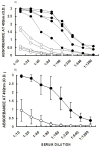
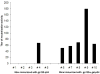
Developing an effective HIV-1 vaccine will require strategies to enhance antigen presentation to the immune system. In a previous study we demonstrated a marked increase in immunogenicity of the highly glycosylated HIV-1 gp120 protein following enzymatic addition of alpha-gal epitopes to the carbohydrate chains. In the present study we determined whether gp120(alphagal) can also serve as an effective platform for targeting other HIV-1 proteins to APC and thus increase immunogenicity of both proteins. For this purpose we produced a recombinant fusion protein between gp120 and the HIV-1 matrix p24 protein (gp120/p24). Multiple alpha-gal epitopes were synthesized enzymatically on the gp120 portion of the fusion protein to generate a gp120(alphagal)/p24 vaccine. Immune responses to gp120(alphagal)/p24 compared to gp120/p24 vaccine lacking alpha-gal epitopes were evaluated in alpha1,3galactosyltransferase knockout (KO) mice. These mice lack alpha-gal epitopes and, therefore, are capable of producing the anti-Gal antibody. T cell responses to p24, as assessed by ELISPOT and by CD8+ T cells intracellular staining assays for IFNgamma, was on average 12- and 10-fold higher, respectively, in gp120(alphagal)/p24 immunized mice than in mice immunized with gp120/p24. In addition, cellular and humoral immune responses against gp120 were higher by 10-30-fold in mice immunized with gp120(alphagal)/p24 than in gp120/p24 immunized mice. Our data suggest that the alpha-gal epitopes on the gp120 portion of the fusion protein can significantly augment the immunogenicity of gp120, as well as that of the fused viral protein which lacks alpha-gal epitopes. This strategy of anti-Gal mediated targeting to APC may be used for production of effective HIV-1 vaccines comprised of various viral proteins fused to gp120.
Published by Elsevier Ltd.
Figures



Similar articles
-
Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R epitopes.J Virol. 2006 Jul;80(14):6943-51. doi: 10.1128/JVI.00310-06. J Virol. 2006. PMID: 16809300 Free PMC article.
-
Amplifying immunogenicity of prospective Covid-19 vaccines by glycoengineering the coronavirus glycan-shield to present α-gal epitopes.Vaccine. 2020 Sep 29;38(42):6487-6499. doi: 10.1016/j.vaccine.2020.08.032. Epub 2020 Aug 19. Vaccine. 2020. PMID: 32907757 Free PMC article. Review.
-
Immune responses against a new HIV-1 p24-gp41/pCAGGS-IL-12 DNA vaccine in Balb/c mice.Iran J Immunol. 2012 Jun;9(2):86-97. Iran J Immunol. 2012. PMID: 22735796
-
Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells.J Virol. 2007 Sep;81(17):9131-41. doi: 10.1128/JVI.00647-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609270 Free PMC article.
-
Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (Review).Int J Mol Med. 2016 Jan;37(1):11-20. doi: 10.3892/ijmm.2015.2397. Epub 2015 Oct 30. Int J Mol Med. 2016. PMID: 26531137 Free PMC article. Review.
Cited by
-
Live unattenuated vaccines for controlling viral diseases, including COVID-19.J Med Virol. 2021 Apr;93(4):1943-1949. doi: 10.1002/jmv.26453. Epub 2020 Sep 29. J Med Virol. 2021. PMID: 32833258 Free PMC article. Review.
-
Marine algal antagonists targeting 3CL protease and spike glycoprotein of SARS-CoV-2: a computational approach for anti-COVID-19 drug discovery.J Biomol Struct Dyn. 2022;40(19):8961-8988. doi: 10.1080/07391102.2021.1921032. Epub 2021 May 20. J Biomol Struct Dyn. 2022. PMID: 34014150 Free PMC article.
-
A cell-free biosynthesis platform for modular construction of protein glycosylation pathways.Nat Commun. 2019 Nov 27;10(1):5404. doi: 10.1038/s41467-019-12024-9. Nat Commun. 2019. PMID: 31776339 Free PMC article.
-
Use of Dendritic Cell Receptors as Targets for Enhancing Anti-Cancer Immune Responses.Cancers (Basel). 2019 Mar 24;11(3):418. doi: 10.3390/cancers11030418. Cancers (Basel). 2019. PMID: 30909630 Free PMC article. Review.
-
In Silico Design and Immunologic Evaluation of HIV-1 p24-Nef Fusion Protein to Approach a Therapeutic Vaccine Candidate.Curr HIV Res. 2018;16(5):322-337. doi: 10.2174/1570162X17666190102151717. Curr HIV Res. 2018. PMID: 30605062 Free PMC article.
References
-
- Bucy RP, Kilby JM. Perspectives on inducing efficient immune control of HIV-1 replication--a new goal for HIV therapeutics? Aids. 2001;15 (Suppl 2):S36–42. - PubMed
-
- Buge SL, Ma HL, Amara RR, Wyatt LS, Earl PL, Villinger F, et al. Gp120-alum boosting of a Gag-Pol-Env DNA/MVA AIDS vaccine: poorer control of a pathogenic viral challenge. AIDS Res Hum Retroviruses. 2003;19(10):891–900. - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5(3):233–6. - PubMed
-
- Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4(8):630–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

